
XPS study of BZT thin film deposited on /Ti/SiO2/Si substrate by pulsed laser deposition
JIANG Yan-ping(蒋艳平)1, 2, TANG Xin-gui(唐新桂)1, LIU Qiu-xiang(刘秋香)1,
CHENG Tie-dong(程铁栋)1, ZHOU Yi-chun(周益春)2
1. School of Physics & Optoelectric Engineering, Guangdong University of Technology,Guangzhou Higher Education Mega Center, Guangzhou 510006, China;
2. Key Laboratory of Low Dimensional Materials and Application Technology, Xiangtan University,Xiangtan 411105, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
Ferroelectric materials were widely applied for actuators and sensors. Barium zirconate titanate Ba(Zr0.25Ti0.75)O3 thin film was grown on Pt/Ti/SiO2/Si(100) substrates by pulsed laser deposition. Structure and surface morphology of the thin film were studied by X-ray diffractometry (XRD) and scan electronic microscopy (SEM). The composition and chemical state near the film surface were obtained by X-ray photoelectron spectroscopy (XPS). On the sample surface, O 1s spectra can be assigned to those from the lattice and surface adsorbed oxygen ions, while C1s only result from surface contamination. The result shows that only one chemical state is found for each spectrum of Ba 3d, Zr 3d and Ti 2p photoelectron in the BZT thin film.
Key words:
chemical state; XPS; BZT thin film; pulsed laser deposition;
1 Introduction
Ferroelectric thin films have been attracted much interest of researchers because of their electronic, optoelectronic and electromechanical applications such as memory devices, field effect transistors, optical and microelectromechanical devices. Many properties of barium strontium titanate ((Ba1-xSrx)TiO3) thin films have been studied, such as high dielectric constant, low leakage current[1, 2]. On the other hand, the barium zirconate titanate (Ba(ZryTi1-y)O3) thin films were considered a promising material substitutes to BST, Because Zr4+ is chemically more stable than Ti4+, Curie temperature can be shifted below room temperature by doping with Zr. Adjusting the Curie temperature can alter characteristics of the BZT film. There are many methods to prepare BZT thin films, such as radio frequency- magnetron sputtering [3-4], the sol-gel method [5-6], and pulsed laser disposition [7-8]. LIANG et al [9] studied the dielectric properties and tunability of BZT ceramics and TANG et al [10] found that the Zr ions substitution of Ti ions had a strong effect on the dielectric properties and the grain sizes. CHOI et al [3, 11] investigated the effect of deposition temperature and cerium doping on the electrical and physical properties of the BZT thin films prepared on the Pt/SiO2/Si substrate with RF magnetron sputter deposition. WU et al [12] and ZHAI et al [13] prepared the strongly preferred orientation BZT thin films by using LaNiO3 as buffer layer on Pt/Ti/SiO2/Si substrate. DIXIT et al [14] studied the phase transition of the BZT thin films.
However, there are fewer reports on the composition and the chemical state of the BZT thin films at the surface and the interface between the thin film and the substrate and they may have effect on the properties of the BZT thin film. This study was devoted to the study of the structure, the composition and the chemical state of barium zirconate titanate Ba(Zr0.25Ti0.75)O3, thin films by X-ray photoelectron spectroscopy (XPS). The thin films were grown on Pt/Ti/SiO2/Si(100) substrates by pulsed laser disposition.
2 ExperimentalBa(ZrxTi1-x)O3 (with x=0.25, abbreviated as BZT25/75) thin film was grown on the Pt(111)/Ti/ SiO2/Si(100) substrates by pulsed laser deposition. The laser source used was a KrF Eximer laser, Lambda Physik Complex with wavelength of λ=248 nm, energy of 650 mJ and pulse duration of 25 ns. The BZT target was prepared from powders synthesized by the sol-gel process[15]. The appropriate proportions of high purity barium acetate, irconium-n-propoxide and titanium n-butoxide were used as precursors. Acetic acid and 2-methoxyethanol were selected as solvents. Barium acetate was dissolved in acetic acid, and the two alkoxides were dissolved in 2-methoxyethanol. By controlling the hydrolysis condition of the complex solution, a BZT gel was formed. The dry gel was annealed at 1 100 ℃ for 5 h in atmosphere, then ground and in this case the BZT powders were obtained. The annealed powders were pressed into disks. The disks were sintered at 1 400 ℃ for 5 h and cooled in a furnace to obtain the BZT target. The films were deposited at a laser repetition rate of 10 Hz and pulsed laser energy of 350 mJ. The base pressure of the vacuum chamber was 0.266 Pa. The deposition rate was 25-30 nm/min, and deposition time was 20 min. The oxygen pressure was an important factor and was kept as 200. Finally, the deposited sample was again heated up to 650 ℃ in 53.2 Pa of oxygen for 10 min and cooled down slowly to room temperature. In this case, the thin films were crystallized in situ. The details of the deposition conditions are given in Table 1.
Table 1 Optimal deposition conditions of BZT thin films
The crystalline structure of the BZT25/75 thin films on Pt/Ti/SiO2/Si substrate was analyzed by Philips PW3710 X-ray diffractometer using Cu Kα radiation with a Ni filter. The surface and cross-sectional morphologies were determined by scanning electron microscopy (SEM, Leica Stereoscan 440). To identify the composition and chemical state near the film surface, X-ray photoelectron spectroscopy (XPS) analyses were carried out on the ESCALAB 250 (VG Scientific) spectrometer by using Mg Kα (1 253.6 eV) radiation.
3 Results and discussion
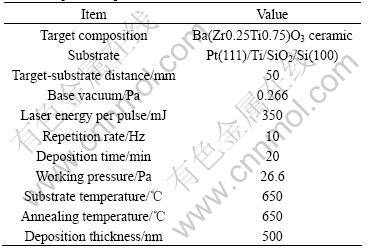
Fig.1 shows the X-ray diffraction pattern of BZT25/75 thin film grown on Pt/Ti/SiO2/Si substrate by pulsed laser deposition. From the XRD pattern, the BZT thin film has polycrystalline structure. There are (100), (110), (111), (200), (210) and (211) six peaks in the XRD pattern. The relative peak intensity of I(111)/SI(khl) is found to be 0.45. It is evident that the film is perovskite phase and has the (111) preferred orientation.
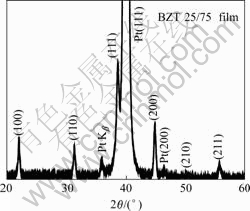
Fig.1 XRD pattern of BZT25/75 film on Pt (111)/Ti/ SiO2/Si(100) substrates
The surface and cross-section morphologies of the film by SEM are shown in Fig.2. According to the images, the microstructure is smooth and dense. The cross-section image presents that the thickness of the film is uniform and it is 500 nm.
Fig.3 shows the wide-scan XPS spectrum of the BZT thin film in the binding energy range from 0 to 1 100 eV. All of the binding energies at various peaks were calibrated by the binding energy of C 1s (284.60 eV) as shown in Fig.4. From the spectrum it is clear that the BZT film contains Ba, Zr, Ti, O and C elements near its surface, and no other impurity element is detected in the spectrum up to 1 100 eV except for carbon. The carbon results from the surface contamination [16]. The quantitative atomic composition near the film surface is determined from the spectra of Ba 3d, Zr 3d, Ti 2p and O1s by using sensitivity factors of 1, 2.1, 1.8 and 0.66, respectively.
Quantitative XPS analysis result not only provides the chemical composition near the sample surface, but also gives the formation on the chemical bonding. From the formation of the chemical bonding, the compounds
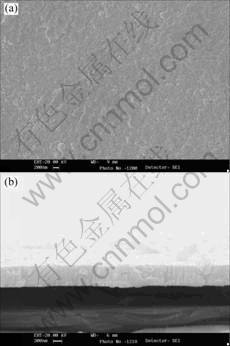
Fig.2 Surface and cross-section morphologies of film: (a) SEM image of surface; (b) Cross-section morphology for BZT25/75 film on Pt/Ti/SiO2/Si film
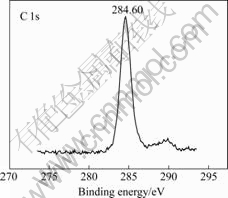
Fig.4 Narrow-scan XPS spectrum of C 1s film
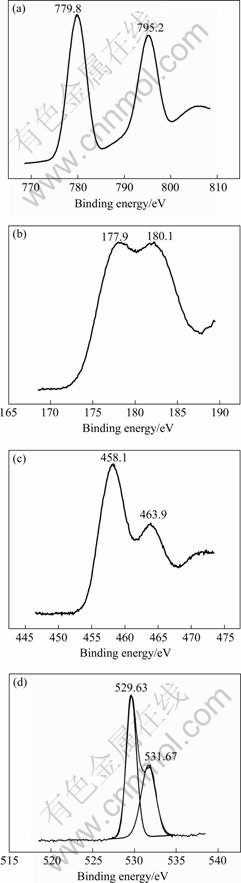
Fig.5 Narrow-scan XPS spectra: (a) Ba 3d; (b) Zr 3d; (c) Ti 2p; (d) O 1s peaks for BZT film
existing near the surface can be determined. The narrow-scan XPS spectra of Ba 3d, Zr 3d, Ti 2p and O 1s peaks for the BZT film are shown in Fig.5. A doublet structure exists in the XPS spectrum of the Ba 3d peak, as shown in Fig.3(a). The Ba 3d5/2 and Ba 3d3/2 peaks separate clearly at 779.8 and 795.2 eV, respectively. The spin orbit splitting between them is 15.4 eV as expected and its full width at half maximum is 4.38 eV.
A doublet structure is also the feature of XPS spectrum of the Zr3 d for BZT film, and clear splitting between Zr 3d5/2 and Zr 3d3/2 peaks can be seen in Fig.3(b). The binding energy of Zr 3d5/2 and Zr 3d3/2 are 177.9 and 180.1 eV, respectively, and the spin-orbit spitting between them is 2.2 eV [16]. The feature of the XPS spectrum of Zi 2p peak is also a doublet structure. The binding energy of Ti 2f3/2 and Ti 2f1/2 are 458.1 and 463.9 eV, respectively, and the spin-orbit spitting between them is 5.8 eV. The full width at half maximum of Ti 2f3/2 peak is about 4.55 eV.
All the XPS spectra of Ba 3d, Zr 3d, Ti 2p of the BZT thin film consist of two peaks corresponding to their angular momentum of electron. Only one spin-orbit doublet is observed for the individual element, i.e. Ba 3d5/2 and Ba 3d3/2 peaks at 779.8 eV and 795.2 eV, Zr 3d5/2 and Zr 3d3/2 peaks at 177.9 eV and 180.1 eV, Ti 2f3/2 and Ti 2f1/2 peaks at 458.1 eV and 463.9 eV. This indicates that only one chemical state exists in the film for each of Ba, Zr and Ti. According to the binding energies, Ba, Zr and Ti ions are considered to be in chemical states of Ba2+, Zr4+ and Ti4+.
Like other elements, a doublet structure is observed in the XPS spectrum of O 1s peak. Its component peak in the spectrum is fitted to a Guassian type distribution with lower binding energy (LBE) and higher binding energy (HBE) peaks at 529.63 eV and 530.67 eV, respectively. The LBE peak is due to the oxide and the HBE peak is due to the hydroxide/absorbed oxygen. The former component is identified as the lattice oxygen of BZT film and the latter as surface adsorbed oxygen.
4 Conclusions
1) BZT25/75 thin film is successfully grown on Pt(111)/Ti/SiO2/Si(100) substrates by pulsed laser deposition. The BZT thin film is well crystallized from the X-ray diffraction (XRD) pattern.
2) The microstructure of the BZT thin film is uniform, dense and no cracks are found by scanning electron microscopy (SEM). The composition and chemical state near the film surface are investigated by X-ray photoelectron spectroscopy (XPS).
3) On the sample surface, O 1s spectrum can be assigned to oxygen ions from the lattice and surface adsorbed oxygen ions, while the C 1s only results from surface contamination.
4) Only one chemical state is found for each spectrum of Ba 3d, Zr 3d and Ti 2p photoelectron in the BZT thin film.
References[1] PONTES F M , LONGO E, LEITE ER, VARELA J A. Study of the dielectric and ferroelectric properties of chemically processed BaxSr1-xTiO3 thin films[J]. Thin Solid Films, 2001, 386: 91-98.
[2] CHO H J, OH S, KANG C S, HWANG C S, LEE B T, LEE K H, HORII H, LEE S I, LEE M Y. Improvement of leakage current characteristics of Ba0.5Sr0.5TiO3 films by N2O plasma surface treatment[J]. Appl Phys Lett, 1997, 71: 3221-3223.
[3] CHOI W S, YI J, HONG B. The effect of cerium doping in barium zirconate titanate thin films deposited by RF magnetron sputtering system[J]. Mater Sci Eng B, 2004, 109: 146-151.
[4] CHOI W S, JANG B S, LIM D G, YI J, HONG B. Characterization of Ba(Zr0.2Ti0.8)O3 thin films deposited by RF-magnetron sputtering[J]. J Crystal Growth, 2002, 237/239: 438-442.
[5] DIXIT A, MAJUMDER S B, SAVVINOV A, KATIYAR R S, GUO R. Investigations on the sol-gel-derived barium zirconium titanate thin films[J]. Mater Lett, 2002, 56: 933-940.
[6] ZHAI Ji-wei, YAO Xi. Strructural and dielectric properties of Ba0.85Sr0.15(Zr0.18Ti0.85)O3 thin films by a sol-gel process[J]. Ceram Int, 2004, 30: 1237-1240.
[7] ZHONG Xiang-li, ZHENG Xue-jun, YANG Jian-tao, ZHOU Yi-chun. Dependence of Ba(Zr0.1Ti0.9)O3 films growth on substrate temperature upon radio-frequency plasma enhanced pulsed laser deposition[J]. J Crystal Growth, 2004, 271: 216-222.
[8] ZHANG Wei, TANG Xing-gui, WONG K H, CHAN H L W. Dielectric properties and high tenability of (100)-oriented Ba(Zr0.2Ti0.8)O3 thin films prepared by pulsed laser deposition[J]. Scripta Mater, 2006, 54: 197-200.
[9] LIANG Rui-hong, DONG Xian-lin, CHEN Ying, CAO eiF, WANG Yong-ling. Dielectric properties and tenability of Ba(ZrxTi1-x)O3 ceramics under high DC electric field[J]. Ceram Int, 2007, 33(6): 957-961.
[10] TANG Xin-gui, WANG J, WANG X X, CHAN H L W. Effects of grain size on the dielectric properties and tunabilities of sol-gel derived Ba(Zr0.2Ti0.8)O3 ceramics[J]. Solid State Commun, 2004, 131: 163-138.
[11] CHOI W S, JANG B S, ROH Y, YI J, HONG B. The effect of temperature on the electrical and physical properties of the Ba(Ti, Zr)O3 thin films[J]. J Non-Cryst Solids, 2002, 303: 190-193.
[12] WU T B, SHY H J, Deposition and properties of highly (100)-orientied barium titanate thin films on LaNiO3 electrode [J]. Ceram Int, 2000, 26: 599-603.
[13] ZHAI Ji-wei, YAO Xi, ZHANG L Y, SHEN Bin. Orientation control and dielectric properties of sol-gel deposited Ba(Ti, Zr)O3 thin films[J]. J Crystal Growth, 2004, 262: 341-347.
[14] DIXIT A, MAJUMDER S B, DOBAL P S, KATIYAR R S, BHALLA A S. Phase transition studies of sol-gel deposited barium zirconate titanate thin films[J]. Thin Solid Films, 2004, 447/448: 284-288.
[15] TANG Xin-gui, CHEW H K, CHAN H L W. Diffuse phase transition and dielectric tunability of Ba(ZryTi1-y)O3 relaxor ferroelectric ceramics[J]. Acta Mater, 2004, 52: 5177-5180.
[16] TANG Xin-gui, DING Ai-li, LUO Wei-gen. Surface morphology and chemical states of highly oriented PbZrO3 thin films prepared by a sol-gel process [J]. Appl Surf Sci, 2001, 174: 148-154.
(Edited by CHEN Can-hua)
Foundation item: Project(05001825) supported by Guangdong Provincial Natural Science Foundation of China; project(KF0707) supported by the Opening Project Program of Key Laboratory of Low Dimensional Materials and Application Technology (Xiangtan University), Ministry of Education, ChinaCorresponding author: JIANG Yan-ping; E-mail: ypjiangzhao@163.com


