Article ID: 1003-6326(2005)05-1185-05
Synthesis and characterization of LiNi0.45Co0.10Mn0.45O2 cathode for lithium ion batteries
GUO Hua-jun(郭华军)1, ZHANG Ming(张 明)1,
LI Xin-hai(李新海)1, ZHANG Xin-ming(张新明)2,
WANG Zhi-xing(王志兴)1, PENG Wen-jie(彭文杰)1, HU Min(胡 敏)1
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. School of Materials Science and Engineering, Central South University, Changsha 410083, China)
Abstract:
LiNi0.45Co0.10Mn0.45O2 was prepared from Li2CO3 and a triple oxide of nickel, cobalt and manganese at 950℃ in air. The structure and characteristics of LiNi0.45Co0.10Mn0.45O2 were determined by XRD, SEM and electrochemical measurements. The compound LiNi0.45Co0.10Mn0.45O2 has layered structure with hexagonal lattice.The individual particles are agglomeration of many little primary particles whose size ranges from 100mm to 200nm. The LiNi0.45Co0.10Mn0.45O2 cathode has excellent electrochemical performances with large reversible specific capacity of 142.5mA·h/g between 4.25V, and good capacity retention of 83.20% after 450 charge/discharge cycles. Capacity of the battery increases with enhancement of charge voltage limit, and a specific discharge capacity of 175.2mA·h/g is obtained when the charge voltage limit is fixed at 4.45V.
Key words:
lithium ion battery; LiNi0.45Co0.10Mn0.45O2; cathode; capacity CLC number: TM912.9;
Document code: A
1 INTRODUCTION
Advanced rechargeable lithium ion batteries are attractive for use in consumer electronic and electric vehicle (EV) application because of a favorable combination of voltage, energy density, cycling performance, and have been developed rapidly worldwide during the past decade[1, 2]. LiCoO2 has been widely used as a cathode material in commercial lithium ion battery because it is reasonable easy to synthesize and shows a stable discharge capacity[3]. But due to its high cost and toxicity, an intensive research for new cathode materials have been underway in recent years[4, 5]. LiNiO2 and LiMnO2 have been extensively studied as possible alternatives to LiCoO2[6, 7]. However, both LiNiO2 and LiMnO2 have problems for practical applications.
A layered LiNi0.5Mn0.5O2 material prepared by co-precipitation method showed promising capacity of around 150mA·h/g in the voltage range of 2.5-4.3V[8, 9]. Kang et al[10] reported increased discharge capacity and electrical conductivity by doping Al, Ti, and Co in LiNi0.5Mn0.5O2. Among those materials, Co doping increased electrical conductivity most successfully. LiNixCoyMn1-x-yO2 is a prospective cathode material due to its low cost and large capacity. Intensive research is conducted on the compounds of LiNi1/3Co1/3Mn1/3O2 and LiNixCo0.05Mn0.95-xO2, and many development has been achieved[11-14].
In this work, a LiNixCoyMn1-x-yO2 compound with new composition of LiNi0.45Co0.10Mn0.45O2 was synthesized by solid-state reaction method, and its structure and electrochemical properties were investigated in detail.
2 EXPERIMENTAL
LiNi0.45Co0.10Mn0.45O2 was prepared from Li2CO3 and a triple oxide of nickel, cobalt, and manganese (Ni∶Co∶Mn=0.45∶0.10∶0.45, Changsha Xinneng Material Co. Ltd). The raw materials were ball milled and well mixed, then roasted at 950℃ for 24h in air. The product was ground into powder using a mortar and pestle. Powder X-ray diffraction(XRD) measurements were made with a Rigaku diffractometer equipped with CuKα radiation and a diffracted-beam monochromator. Scanning electron microscopy(SEM) images were obtained with a Hitachi S530 spectrometer.
The LiNi0.45Co0.10Mn0.45O2 compound was mixed with acetylene black as electric conductor and poly (vinylidene difluoride )(PVDF) as binder. The LiNi0.45Co0.10Mn0.45O2 cathode was prepared by spreading the above mixture on aluminum foil. The preparation of carbon electrode was the same as that of LiNi0.45Co0.10Mn0.45O2 electrode except graphite as active material and copper foil as current collector. The electrolyte was 1mol/L LiPF6 in a 1∶1 mixture of ethylene carbonate(EC) and dimethyl carbonate(DMC). Prototype prismatic batteries of 053048 size were prepared by assembling the LiNi0.45Co0.10Mn0.45O2 cathodes, carbon anodes, electrolyte and Celgard 2300 membrane into stainless-steel cases. The charge/discharge characteristics and cycling performance of prototype batteries were investigated. The prototype batteries were charged in a CC/CV (constant current/constant voltage) pattern, by which the batteries were charged to a fixed voltage at a constant current density, and followed by the voltage held at the fixed voltage until the current density decreased to 5mA/g for LiNi0.45Co0.10Mn0.45-O2. Then the batteries were discharged at a constant current density to 2.75V.
3 RESULTS AND DISCUSSION
3.1 Synthesis and structure characterization of LiNi0.45Co0.10Mn0.45O2
LiNi0.45Co0.10Mn0.45O2 was synthesized by solid state reaction of Li2CO3 with a triple oxide of nickel, cobalt and manganese at 950℃ in air. The XRD pattern of the compound LiNi0.45Co0.10Mn0.45-O2 shown in Fig.1 is similar to that of LiCoO2 (α-NaFeO2 type, space group R3[TX-]m) and can be indexed as hexagonal lattice. The transition metal atoms (M=Ni, Co, Mn) are supposed to be randomly distributed on the 3b sites, whereas the lithium atoms occupy the 3a sites and O atoms occupy the 6c sites. A partial interchange of occupancy of Li and transition metal atoms among the sites (i.e. Li on 3b and M on 3a sites) would give rise to disordering in the structure called ‘cation mixing’. The integrated intensity ratio of the (003) to (104) lines (R) in the XRD patterns was shown to be a measure of the cation mixing[15]. As shown in Fig.1, the (003) peak is higher and narrower than (104) peak. The ratio of integrated intensities of the (003) to (104) lines is only 0.938, well below the values (R>1.2) reported for compounds like Li(Ni1-xCox)O2 and LiNiO2, which indicates obvious cation mixing in the microstructure of the LiNi0.45Co0.10Mn0.45O2 as prepared[15, 16].
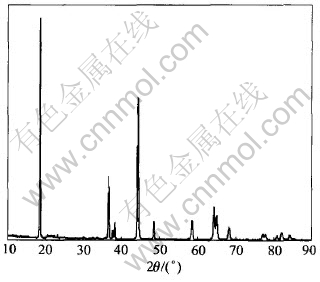
Fig.1 XRD pattern of LiNi0.45Co0.10Mn0.45O2

Fig.2 SEM micrographs of LiNi0.45Co0.10Mn0.45O2
Fig.2 shows the scanning electron micrographs for the LiNi0.45Co0.10Mn0.45O2 powders. The sample consists of cornered and uneven particles with rough surfaces and an average particle size of about 30μm. There are some tiny particles clung to the large ones. As shown in Fig.2(b), the individual particles are composed of many little primary particles whose size ranges from 100nm to 200nm. The primary particles reunite each other and form secondary agglomeration, and many narrow cracks are observed among the primary particles.
3.2 Electrochemical characteristics of Li-ion battery with LiNi0.45Co0.10Mn0.45O2 cathode
Electrochemical characteristics of the battery with LiNi0.45Co0.10Mn0.45O2 cathode have been evaluated. Laid for 24h after being assembled, the battery has an open circuit voltage of 0.126V. Fig.3(a) shows the initial charge-discharge voltage profiles for the battery at a current density of 30mA/g for LiNi0.45Co0.10Mn0.45O2. On starting the current, the voltage of the cell suddenly rises to 3.0V, then slowly increases to 4.20V. The battery shows an initial discharge capacity of 135.1mA·h/g for LiNi0.45Co0.10Mn0.45O2 with initial coulumbic efficiency of 83.68%. After several charge/discharge cycles, the capacity of the battery increases slowly and a reversible capacity of 142.5mA·h/g for LiNi0.45Co0.10Mn0.45O2 is observed in the voltage of 2.75-4.25V.

Fig.3 Initial charge/discharge characteristics of Li-ion battery with LiNi0.45Co0.10Mn0.45O2 cathode
To illustrate the process more clearly, the relationship between differential capacity (dC/dV) and voltage are given in Fig.3(b). It shows two differential capacity peaks for the charge process with the first sharp and intense peak centered at 3.816V and the second peak at 4.156V. However, there is only a low intensity and broad differential capacity peak at 3.682V for the discharge process. It can be observed that the discharge differential capacity peak shifts obviously to lower voltage from the two charge differential capacity peaks. This is caused by the polarization and low electronic conductivity of electrode materials and electrolyte.
The dependence of capacity on the charge voltage limit for the Li-ion battery with LiNi0.45Co0.10-Mn0.45O2 cathode is determined. The Lithium ion battery was charged to 4.20V at a constant current density of 30mA/g LiNi0.45Co0.10Mn0.45O2 and followed with a constant voltage of 4.20V until the charge current density decreased to 5mA/g LiNi0.45Co0.10Mn0.45O2, then the battery was charged repeatedly in the same patterns except the charge voltage limit is fixed at 4.25, 4.30, 4.35, 4.40 and 4.45V, respectively. The voltage profiles and the dependence of charge capacity on the charge voltage limit are shown in Fig.4. When the charge voltage limit is enhanced, the charge capacity of the battery increases obviously, with 135.3mA·h/g for 4.20V and 189.4mA·h/g for 4.45V, respectively. Having been charged to 4.45V, the battery is discharged at a current density of 30mA/g for LiNi0.45Co0.10Mn0.45O2 to 2.75V and a specific discharge capacity of 175.2mA·h/g for LiNi0.45Co0.10Mn0.45O2 with a new irreversible capacity of 14.2mA·h/g is observed. This part of capacity loss may be due to some irreversible reaction of the electrolyte with electrodes at high voltage.
The rate-capability of battery with LiNi0.45-Co0.10Mn0.45O2 electrode was examined and the results are shown in Fig.5. The cell was charged to 4.25V at a constant current density of 75mA/g LiNi0.45Co0.10Mn0.45O2 and followed with a constant voltage of 4.25V until the charge current density decreased to 5mA/g, then discharged at a constant current density of 15-150mA/g corresponding to 0.1-1C rate. The discharge capacity of the LiNi0.45Co0.10Mn0.45O2 cathode at 0.1C rate (15mA/g) is 142.7mA·h/g. When the cell is discharged at 1C rate, 89.25% capacity is observed. Fig.5(b) shows dC/dV vs voltage profiles at different discharge current rates for the battery with LiNi0.45Co0.10Mn0.45O2 cathode. With the increase of discharge current rate, the differential capacity peak shifts to lower voltage and its intensity decreases. It is resulted from the larger polarization and resistance when the battery is discharged at higher current density.
Fig.6 shows cycling characteristics of the lithium ion battery charged/discharged in the voltage range of 2.75-4.25V at 0.5C rate (75mA/g LiNi0.45Co0.10Mn0.45O2). The discharge capacity of the battery increases a little during the early several cycles, then decreases slowly and remains 83.20% of the initial capacity after 450 charge/discharge cycles, which indicates that the battery with LiNi0.45Co0.10Mn0.45O2 cathode has excellent cycling performance.

Fig.4 Relationship between capacity and charge voltage limit for Li-ion battery with
LiNi0.45Co0.10Mn0.45O2 cathode

Fig.5 Characteristics of Li-ion battery with LiNi0.45Co0.10Mn0.45O2 cathode at
different discharge current rates

Fig.6 Cycling performance of Li-ion battery with LiNi0.45Co0.10Mn0.45O2 cathode
4 CONCLUSIONS
1) Layered structure LiNi0.45Co0.10Mn0.45O2 was synthesized from Li2CO3 and a triple oxide of nickel, cobalt and manganese by solid state reaction method.The LiNi0.45Co0.10Mn0.45O2 sample consists of cornered and uneven particles with an average particle size of around 30μm. The individual particles are agglomeration of many little primary particles which range from 100nm to 200nm.
2) The LiNi0.45Co0.10Mn0.45O2 in practical Li-ion batteries shows an initial discharge capacity of 135.1mA·h/g with initial coulumbic efficiency of 83.68% in the voltage range of 2.75- 4.20V. Capacity of the battery increases with enhancement of charge voltage limit, and a specific discharge capacity of 175.2mA·h/g is obtained when the charge voltage limit is fixed at 4.45V.
3) The LiNi0.45Co0.10Mn0.45O2 cathode has excellent electrochemical performances with large reversible specific capacity of 142.5mA·h/g between 2.75V and 4.25V, and good capacity retention of 83.20% after 450 charge/discharge cycles, which indicates LiNi0.45Co0.10Mn0.45O2 is a promising alternative material to LiCoO2 for cathode of lithium ion batteries.
REFERENCES
[1]Jongh P E, Notten P H L. Effect of current pulse on lithium intercalation batteries [J]. Solid State Ionics, 2002, 148: 259-268.
[2]GUO Hua-jun, LI Xin-hai, WANG Zhi-xing, et al. Si-doped composite carbon as anode of lithium ion batteries [J]. Trans Nonferrous Met Soc China, 2003, 13(5): 1062-1065.
[3]Bok J S, Lee J H, Lee B K. Effect of synthetic conditions on electrochemical activity of LiCoO2 prepared from recycled cobalt compounds [J]. Solid State Ionics, 2004, 169: 139-144.
[4]Lee C W, Sun Y K, Prakash J. A novel layered Li-[Li0.12NizMg0.32-zMn0.56]O2 cathode material for lithium ion batteries [J]. Electrochimica Acta, 2004, 49: 4425-4432.
[5]LI Hui, ZHAI YU-chun, TIAN Yan-wen, Preparation of LiNi1-yCoyO2 in certain oxygen pressure [J]. Trans Nonferrous Met Soc China, 2003, 13(5): 1046-1050.
[6]Chang C C, Kim J Y, Kumta P N. Influence of crystallite size on the electrochemicall properties of chemically synthesized stoichoimetric LiNiO2 [J]. J Electrochem Soc, 2002, 149(9): A1114-A1120.
[7]Cha J, Kim T J, Park B, The effect of a metal oxide coating on the cycling behavior at 55℃ in orthorhomibic LiMnO2 cathode materials [J]. J Electrochem Soc, 2002, 149(3): A288-A292.
[8]Sun Y K, Yoon C S, Lee Y S. Electrochemical properties and structural characterization of layered Li[Ni0.5Mn0.5]O2 cathode materials [J]. Electrochimica Acta, 2003, 48: 2589-2592.
[9]Shaju K M, Subba R G V, Chowdari B V R. Li-ion kinetics and polarization effect on the electrochemical performance of Li(Ni1/2Mn1/2)O2 [J]. Electrochimica Acta, 2004, 49: 1565-1576.
[10]Kang S H, Amine K, Comparative of Li(Ni0.5-x-Mn0.5-xM2x)O2 cathode materials for rechargeable lithium batteries [J]. J Power Sources, 2003, 119: 150-155.
[11]Yoshio M, Noguchi H, Itoh J, et al. Preparation and properties of LiCoyMnxNi1-x-yO2 as a cathode for lithium ion batteries [J]. J Power Sources, 2000, 90: 176-181.
[12]Yabuuchi N, Ohzuku T. Novel lithium insertion material of Li(Ni1/3Co1/3Mn1/3)O2 for advanced lithium-ion batteries [J]. J Power Sources, 2003, 119: 171-174.
[13]Park S H, Yoon C S, Kang S G, et al. Synthesis and structural characterization of layered Li(Ni1/3Co1/3-Mn1/3)O2 cathode materials by ultrasonic spray pyrolysis method [J]. Solid State Ionics, 2004, 171: 167-172.
[14]Kim J H, Park C W. Synthesis and electrochemical behavior of Li[Li0.1Ni0.35-x/2CoxMn0.55-x/2]O2 cathode materials [J]. Solid State Ionics, 2003, 164: 43-49.
[15]Chowdari B V R, Subba R G V, Chow S Y. Cathodic behavior of (Co, Ti, Mg)-doped LiNiO2 [J]. Solid State Ionics, 2001, 140: 55-62.
[16]Shaju K M, Subba R G V, Chowdari B V R. Performance of layered Li(Ni1/3Co1/3Mn1/3)O2 as cathode for Li-ion batteries [J]. Electrochimica Acta, 2002, 48: 145-151.
Foundation item: Project(50302016) supported by the National Natural Science Foundation of China and project(2005037698) supported by Postdoctoral Science Foundation of China
Received date: 2005-04-15; Accepted date: 2005-07-21
Correspondence: GUO Hua-jun, Associate professor, PhD; Tel: +86-731-8836633; E-mail: ghj@mail.csu.edu.cn


