- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discussion▲
- 4 Conclusions▲
- References
- Figure
- Fig.1 Zinc extraction efficiency of marmatite with different bacteria groups
- Fig.2 Variations of cell densities in leachates during processes of marmatite leaching with different bacteria groups
- Fig.3 Changes of pH (a) and Eh (b) values of leachate vs time during processes of marmatite leaching with different bacteria groups
- Fig.4 Zinc extraction efficiency of marmatite at different venting levels
- Fig.5 Cell densities of leachate during leaching process of marmatite with different venting levels
- Fig.6 Changes of pH (a) and Eh (b) values of leachate vs time during processes of marmatite leaching with different venting levels
- Fig.7 Variations of zinc concentration (a) and extraction efficiency (b) vs time at different pulp densities
- Fig.8 Changes of pH (a) and Eh (b) values of leachate vs time at different pulp densities
- Fig.9 Cell densities of leachate vs time at different pulp densities
J. Cent. South Univ. Technol. (2008) 15: 650-655
DOI: 10.1007/s11771-008-0121-9
![]()
Bioleaching of marmatite using moderately thermophilic bacteria
ZHOU Hong-bo(周洪波)1, 2, LIU Fei-fei(刘飞飞)1, ZOU Ying-qin(邹颖钦)1,
ZENG Xiao-xi(曾晓希)1, QIU Guan-zhou(邱冠周)1, 2
(1. School of Resources Processing and Bioengineering, Central South University,
Changsha 410083, China;
2. Key Laboratory of Biometallurgy, Ministry of Education, Central South University,
Changsha 410083, China)
Abstract:
The process of bioleaching marmatite using moderately thermophilic bacteria was studied by comparing marmatite leaching performance of mesophiles and moderate thermophiles and valuating the effect of venting capacity as well as pulp density on marmatite leaching performance of moderate thermophiles. The results show that moderate thermophiles have more advantages over mesophilies in bioleaching marmatite at 45 ℃ and the pulp density of 50 g/L, and the zinc extraction efficiency reaches 93.1% in 20 d. Aeration agitation can improve the transfer of O2 and CO2 in solution and promote the growth of bacteria and therefore, enhance the leaching efficiency. Under the venting levels of 50, 200 and 800 mL/min, the zinc extraction efficiencies by moderate thermophiles are 57.8%, 92.5% and 96.0%, respectively. With the increase of pulp density, the total leaching amount of valuable metals increases, however, the extraction efficiency decreases due to many reasons, such as increasing shear force leading to poorly growth condition for bacteria, etc. The zinc extraction decreases remarkably to 58.9% while the pulp density mounts up 20%.
Key words:
marmatite; moderate thermophilies; zinc; bioleaching;
1 Introduction
China has zinc storage capacity of 75×106 t, which accounts for 25% of the world gross storage and ranks second in the world. Among available zinc storage capacity of 33×106 t, a high percentage is rich in marmatite. Due to the concomitance of zinc ore and other metals like Pb, Sn, Ag, Co, etc, in the process of exploiting the concomitant metals, by-products rich in marmatite are yielded[1]. It proves difficult to treat marmatite by conventional extraction processes due to the high content of iron as well as the little economic benefit. Comparatively, bioleaching has received increasing attention due to the advantages of maneuverability, low cost, low energy consumption and, most important, high utilization of resources and environment friendliness. With further research, bioleaching will be increasingly used in extraction of ore concentrate, and applied extensively in the pre-process of difficult-to-process gold ores and processing of Zn, Ni, Co and other types of sulfide minerals[2].
Dozens of bacterial species have been identified as having bioleaching capabilities, including chemoautotroph bacteria, heterotrophic bacteria, fungi, etc. According to the optimal growth temperature, those bacteria can be categorized into mesophiles (30 ℃), moderate thermophiles (50 ℃), and extreme thermo- philes (70 ℃). Traditionally, close attention has been paid to the mesophilic bacteria, especially Acidithioba- cillus ferrooxidans (At. ferrooxidans). It has been proved that At. ferrooxidans has the capacity of bioleaching of sulphide minerals like Zn[3-4], Co[5-6], Ni[7-8], etc. However, At. ferrooxidans also has the disadvantage of low efficiency of dynamics in bioleaching, which restricts its industry application. Recent researches show that moderate thermophilic bacteria have superiority on leaching dynamics in the bioleaching of complex of lead and zinc sulphide, which attracts our attention[9-10]. SHI and FANG[11] reported that moderate thermophilic bacteria could work at a higher temperature and lower pH compared to At. ferrooxidans and achieved higher zinc extraction efficiency. Moreover, using the mixture of several species can improve the extraction rate of valuable minerals[12].
In this work, moderate thermophiles were used to leach marmatite and the bioleaching capabilities were compared between moderately thermophilic bacteria and mesophilic bacteria. The effects of venting and ore concentration were investigated in the bioleaching of marmatite with moderate thermophiles in order to provide knowledge for further research and industrial application.
2 Experimental
2.1 Mineral
The marmatite used in the experiments was not processed by flotation. The sample contained 48.38% Zn, 1.10% Fe and 20.2% S (mass fraction), and was ground to particle size less than 75 μm. The 9k inorganic salt medium was used as leaching solution with initial pH value of 1.9[13].
2.2 Microorganisms
The moderate thermophiles used in this work were collected in the author’s laboratory from hot spring, ore, acid mine drainage in provinces of Yunnan, Jiangxi, Hunan, Guangdong and Gansu, etc. Community analysis by PCR-RFLP showed that it mainly consisted of Sulfobacillus thermotolerans (S. thermotolerans), Sulfo- bacillus thermosulfidooxidans (S. thermosulfidooxidans), At. caldus, Leptospirillum ferriphilum (L. ferriphilum). Mesophilic bacteria used in this research were the product of mixing after isolating from acidophilic mesophilic bacteria, which mainly contained At. ferrooxidans and At. thiooxidans.
2.3 Experimental process
In 250 mL Erlenmeyer flasks, the marmatite sample was added into 120 mL 9k medium to concentration of 50 g/L. Each flask was inoculated with 20 mL (1×108 /mL) moderate thermophilic culture and mesophilic culture respectively. By adjusting the pH value to 1.9, the flasks were shaken on the orbital shakers at 160 r/min and growth temperatures of 45 and 30 ℃, respectively. The volume reduced by evaporation was compensated by sterilized water, and pH, redox potential Eh (vs SCE) and bacterial density were measured everyday. Zn concentration in the supernatant was analyzed every other day.
In 250 mL Erlenmeyer flasks, the marmatite sample was added into 180 mL 9k medium to concentration of 50 g/L. Each flask was inoculated with 20 mL moderately thermophilic bacteria. The pH value was adjusted to 1.9, and the flasks were controlled at 45 ℃ in thermostatic waterbath. To study the effect of venting capacity on marmatite leaching performance, the venting flowrates were adjusted to 50, 200 and 800 mL/min, respectively.
In 250 mL Erlenmeyer flasks, the marmatite sample was added into 120 mL 9k medium to concentrations of 50 g/L, 100 g/L and 200 g/L, respectively. Each flask was inoculated with 20 mL moderately thermophilic bacteria. The pH value was adjusted to 1.9, and the flasks were shaken on the orbital shakers at 160 r/min and 45 ℃.
2.4 Analysis methods
Elemental analysis: the concentrations of Zn and Fe in the leaching solution were measured by atomic absorption spectrometry (AAS). The pH value and Eh value in the leaching solution were measured by a pH S-3C acid meter and a platinum electrode with an Ag/AgCl reference electrode, respectively. Free cells in solution were observed and counted under an optical microscope.
3 Results and discussion
3.1 Comparison of marmatite leaching with mesophilic bacteria and moderately thermophilic bacteria
Under the condition of 160 r/min and 50 g/L pulp density, the moderately thermophilic bacteria show higher leaching efficiency than mesophilic bacteria in a leaching period of 20 d (Fig.1). At 45 ℃, compared with the zinc extraction efficiency of 3.1% in the sterile control, the zinc extraction efficiency of moderately thermophilic bacteria is 93.1%. At 30 ℃, the zinc extraction of mesophilic bacteria is 57.0%, while the value is 2.6% in the sterile control.
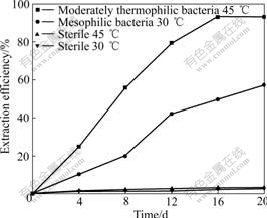
Fig.1 Zinc extraction efficiency of marmatite with different bacteria groups
At the beginning, the cell densities of mesophilic culture and thermophilic culture increase fast until 10th day when the densities become stable (Fig.2). The cell density (shown as logarithm of the cell number, ρc) of moderately thermophilic bacteria is greater than that of mesophilic bacteria at 20th day, and the maximum cell densities are 3.64×108 /mL and 2.88×108 /mL, respectively.
The variations of pH value and Eh under different conditions (Fig.3) indicate that pH value is generally increasing both in thermophilic and mesophilic sterile conditions, which may be due to the dissolution of marmatite in the acid leaching solution, thus leading to the release of the alkaline substances. While in the condition with bacteria added, pH values in leaching solution have a transient increase then decrease quickly, and the decrease in moderately thermophilic bacteria solution is more significant, and the final pH value is only 0.8. Eh values also increase after being inoculated with bacteria, and the redox potentials increase from 278 (vs SCE) to 552 mV (vs SCE), and from 278 (vs SCE) to 500 mV (vs SCE) for thermophilic and mesophilic cultures, respectively. However, Eh values in both sterile systems decrease from 320 (vs SCE) to around 220 mV (vs SCE).
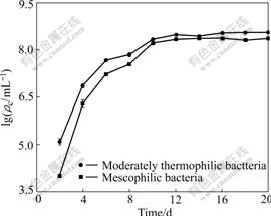
Fig.2 Variations of cell densities in leachates during processes of marmatite leaching with different bacteria groups
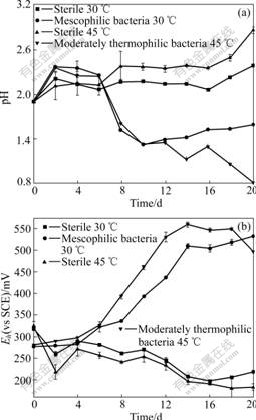
Fig.3 Changes of pH (a) and Eh (b) values of leachate vs time during processes of marmatite leaching with different bacteria groups
Similar to the results reported by DEVECI et al[10], under the condition of shaking, moderately thermophilic bacteria have a superior quality over mesophilic bacteria in bioleaching. At 45 ℃, the higher cell density and stronger metabolic ability cause lower pH value that increases the dissolution of marmatite and improves the direct and indirect effects of mineral leaching and elevates the zinc extraction.
3.2 Effect of venting capacity on marmatite leaching performance of moderately thermophilic bacteria
The influence of venting level on the marmatite leaching is obvious. Venting levels of 200 and 800 mL/min result in good agitation, venting level of 50 mL/min, however, results in low pulp density because the low venting level fails to promote all the slurry with part of ore powder deposited at the bottom. At pulp density of 50 g/L and venting levels of 50, 200 and 800 mL/min, the zinc extraction efficiencies by moderately thermophilic bacteria in 20 d are 57.8%, 92.5% and 96.0%, respectively (Fig.4). The cell densities are also influenced by aeration, the highest values for 800 and 50 mL/min venting levels are 1.1×109 and 8.5×108 /mL (Fig.5), respectively.
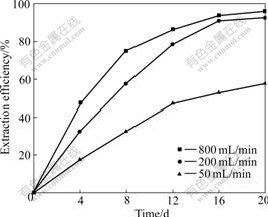
Fig.4 Zinc extraction efficiency of marmatite at different venting levels
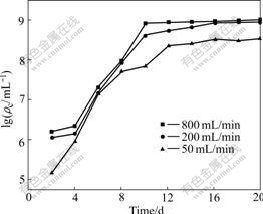
Fig.5 Cell densities of leachate during leaching process of marmatite with different venting levels
Variations of pH and Eh values at different venting levels are shown in Fig.6. The final pH values are 1.11, 0.75 and 0.72 for aeration levels of 50, 200 and 800 mL/min, respectively. Eh value increases in general. It shows higher Eh values at aeration level of 800 mL/min, and the final Eh value is up to 625 mV (vs SCE).
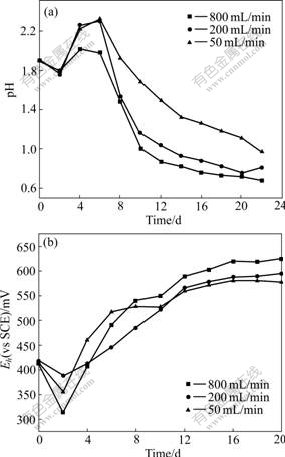
Fig.6 Changes of pH (a) and Eh (b) values of leachate vs time during processes of marmatite leaching with different venting levels
Moderately thermophilic culture mainly consists of S. thermotolerans, S. thermosulfidooxidans, A. caldus and L. ferriphilum that are all aerobic bacteria, therefore, aeration has significant influence on the growth of bacteria and thus plays an important role in dissolution of ores. Strong aeration can improve the biomass, lower the pH value and increase the redox potential of solution, which results in better bioleaching performance. However, too much aeration will cause strong shear force that may damage the cells, interfere with adhesion of bacteria to mineral surface and improve the cost as well.
3.3 Effect of pulp density on marmatite leaching performance of moderately thermophilic bacteria
The results of zinc dissolution at different pulp densities are shown in Fig.7. After 20 d, the zinc extraction efficiencies at pulp densities of 50, 100 and 200 g/L are 93.1%, 81.9% and 58.9%, respectively. With the increase of pulp density, the total amount of dissolved valuable metals increase, however, the extraction efficiency decreases, which conforms with the results made by BAILEY and HANSFORD[14] in a pyrite bioleaching experiment. This is mainly because the dissolution of minerals is primarily limited to the specific surface area of mineral in per unit volume of solution; also, it is limited to CO2 concentration[15]. At higher pulp density, the system has larger specific surface area that increases the time of mineral dissolution and decreases the extraction efficiency. Meanwhile, with the increase of pulp density, the transfer of O2 and CO2 is restricted and the damage of shear force and metal ions to cells increases[16].
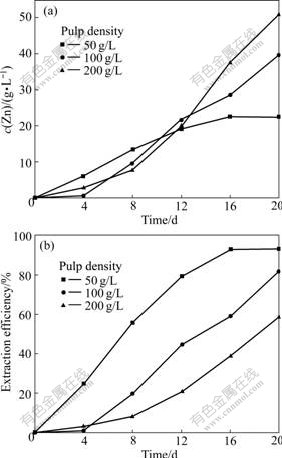
Fig.7 Variations of zinc concentration (a) and extraction efficiency (b) vs time at different pulp densities
The changes of pH and Eh values of leachate at different pulp densities are shown in Fig.8. The pH values in leaching system with pulp density of 50 g/L drop down most, the final pH is 0.81, while Eh values in leaching system with pulp density of 50 g/L rise most, the final Eh values in leaching systems with 50, 100 and 200 g/L pulp densities are 561, 538 and 474 mV (vs SCE), respectively. These are consistent with the above results, which may indicate that lower pH value and higher Eh value are beneficial to marmatite dissolution.
The cell densities are also influenced by pulp density. And the results are shown in Fig.9. It can be seen that the maximum of cell densities at pulp densities of 50, 100 and 200 g/L are 8.6×108, 8.3×108 and 8.0×108 /mL, respectively.
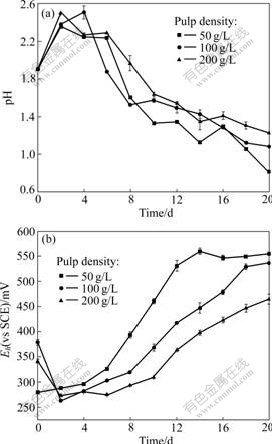
Fig.8 Changes of pH (a) and Eh (b) values of leachate vs time at different pulp densities
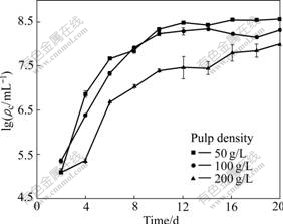
Fig.9 Cell densities of leachate vs time at different pulp densities
4 Conclusions
1) Moderately thermophilic bacteria have more advantages over mesophilic bacteria in bioleaching of marmatite.
2) Stronger aeration is more beneficial to dissolve the marmatite, improve the transfer of O2 and CO2 in solution, promote the growth of bacteria and therefore, enhance the leaching performance.
3) Pulp density has a significant effect on zinc extraction: higher zinc extraction can be obtained at low pulp density, while improving the pulp density can increase the amount of leached zinc, whereas, it may cut down the transfer of O2 and CO2 and increase the shear force, and therefore, decrease the recovery of Zn.
References
[1] DONG Ying. Probe into processing the zinc sulphide concentrate bearing high content of iron [J]. Yunnan Metallurgy, 2000, 29(4): 26-29. (in Chinese)
[2] EHRLICH H L. Past, present and future of bioleaching [J]. Hydrometallurgy, 2001, 59(2/3): 127-134.
[3] GARCIA O J, BIGHAM J M, TUOVIEN O H. Sphalerite oxidation by Thiobacillus ferrooxidans and Thiobacillus thiooxidans [J]. Can J Microbiol, 1995, 41(7): 578-584.
[4] BOON M, SNIJDER M, HANSFORD G S, HEIJNEN J J. The oxidation kinetics of zinc sulphide with Thiobacillus ferrooxidans [J]. Hydrometallurgy, 1998, 48(2): 171-186.
[5] ZAWA H, SATO H. Bacterial leaching of cobalt-rich ferro- manganese crusts [J]. Int J Miner Process, 1995, 43(3/4): 255-265.
[6] BATTAGLIA F, MORIN D, OLLIVIER P. Dissolution of cobaltiferous pyrite by Thiobacillus ferrooxidans and Thiobacillus thiooxidans: Factors influencing bacterial leaching efficiency [J]. J Biotechnology, 1994, 32(1): 11-16.
[7] MASON L J, RICE N M. The adaptation of Thiobacillus ferrooxidans for the treatment of nickel-iron sulphide concentrates [J]. Mineral Engineering, 2002, 15(11): 795-808.
[8] ZHANG Guang-ji, FANG Zhao-heng. Behavior of Fe and S in bioleaching of pentlandite [J]. Trans Nonferrous Met Soc China, 2002, 12(1): 160-163.
[9] TUOVINEN O H, BHATTI T M, BIGHAM J M, HALLBERG K B, GARCIA O Jr, LINDSTR?M E B. Oxidative dissolution of arsenopyrite by mesophilic and moderately thermophilic acidophiles [J]. Appl Environ Microbiol, 1994, 60(9): 3268-3274.
[10] DEVECI H, AKCIL A, ALP I. Bioleaching of complex zinc sulphide using mesophilic and thermophilic bacteria: Comparative importance of pH and iron [J]. Hydrometallurgy, 2004, 73 (3/4): 293-303.
[11] SHI Shao-Yuan, FANG Zhao-heng. Comparison of bioleaching of marmatite flotation concentrates with Acidithiobacillus ferrooxidans and a moderately thermophilic strain [J]. The Chinese Journal of Process Engineering, 2005, 5(4): 384-388. (in Chinese)
[12] WU Chang-bing, ZENG Wei-ming, ZHOU Hong-bo, FU Bo, HUANG Ju-fang, QIU Guan-zhou, WANG Dian-zuo. Bioleaching of chalcopyrite by a mixed culture of moderately thermophilic microorganisms [J]. Journal of Central South University of Technology, 2007, 14(4): 474-478.
[13] ZHOU Hong-bo, LIU Xi, FU Bo, HUO Qiang, ZENG Wei-ming, LIU Jian-she, QIU Guan-zhou, CHEN X H. Isolation and characterization of Acidithiobacillus caldus from several typical environments in China [J]. Journal of Central South University of Technology, 2007, 14(2): 163-169.
[14] BAILEY A D, HANSFORD G S. Oxygen mass transfer limitation of batch bio-oxidation at high solids concentration [J]. Minerals Engineering, 1994, 7(2/3): 293-303.
[15] TORMA A E, WALDEN C C, DUNCAN D L W, BRANION R M R. The effect of carbon dioxide and particle surface area on the microbiological leaching of a zinc sulfide concentrate [J]. Biotechnology and Bioengineering, 1972, 14(5): 777-786.
[16] SHI Shao-yuan, FANG Zhao-heng. Bioleaching of marmatite concentrate in magnetic stirring reactor [J]. Nonferrous Metals, 2005, 57(2): 62-65. (in Chinese)
Foundation item: Projects(50621063, 40646029) supported by the National Natural Science Foundation of China; Project(2004CB619204) supported by the Major State Basic Research Development Program of China; Project(NCET-06-0691) supported by the Program for New Century Excellent Talents in University
Received date: 2008-03-15; Accepted date: 2008-05-20
Corresponding author: ZHOU Hong-bo, Professor, PhD; Tel: +86-731-8877216; E-mail: zhouhb@mail.csu.edu.cn
- Bioleaching of marmatite using moderately thermophilic bacteria


