Trans. Nonferrous Met. Soc. China 30(2020) 1687-1696
Behavior of vanadium during reduction and smelting of vanadium titanomagnetite metallized pellets
Shuai WANG, Yu-feng GUO, Fu-qiang ZHENG, Feng CHEN, Ling-zhi YANG, Tao JIANG, Guan-zhou QIU
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 10 October 2019; accepted 9 April 2020
Abstract:
The effects of CaO content, MgO content and smelting temperature on the vanadium behavior during the smelting of vanadium titanomagnetite metallized pellets were investigated. The thermodynamics of reduction and distribution of vanadium was analyzed and the high-temperature smelting experiments were carried out. The thermodynamic calculations show that the distribution ratio of vanadium between the slag and the hot metal decreases with the increments of CaO and MgO content in the slag as well as the increase of the smelting temperature. The smelting experiments demonstrate that the vanadium content in iron and the recovery rate of vanadium in pig iron increase as the CaO content, MgO content and smelting temperature increase, whereas the vanadium distribution ratio between the slag and iron tends to decrease. Moreover, the recovery rate of vanadium in pig iron has a rising trend with increasing the optical basicity of the slag. The addition of MgO in the slag to increase the slag optical basicity can not only improve the vanadium reduction but also promote the formation of magnesium-containing anosovite, which is beneficial to following titanium extraction.
Key words:
vanadium; vanadium titanomagnetite; vanadium distribution ratio; electric-furnace titanium slag; MgO;
1 Introduction
Vanadium titanomagnetite ore serves as an important feedstock for production of vanadium [1]. Currently, the blast furnace (BF) process and the direct reduction-electric furnace (DR-EF) process have been commercialized to recover iron and vanadium, but titanium resource cannot be recovered cost-effectively due to low TiO2 contents of slags [1-4]. Compared with the BF process, the DR-EF process is easier to control for the reduction of oxides because the reductant addition can be accurately adjusted in electric furnace, and the DR-EF process has the advantages such as environmental friendliness and good quality of productions [2,3]. Therefore, the DR-EF process should be developed to produce titanium slag for following titanium extraction [5-10].
In the DR-EF smelting process, vanadium oxides and iron oxides are reduced to molten iron, while titanium oxides are enriched in slag. Vanadium-bearing molten iron is oxidized to produce semisteel and vanadium slag; then vanadium is extracted from the vanadium slag by hydrometallurgy methods [1-4,11,12]. Thus, reduction and distribution behaviors of vanadium during the smelting of vanadium titanomagnetite by the DR-EF process are crucial for subsequent vanadium recovery.
Reduction and distribution behaviors of vanadium between slag and hot metal have been reported in many previous studies [13-26]. JUNG et al [16] and SHIN et al [17] indicated that the vanadium distribution ratio between slag and molten iron increased with the increase of slag basicity but decreased with the rise of temperature. However, WANG et al [23] showed that the distribution ratio of vanadium between titanium slag and molten iron decreased as the basicity increased from 0.35 to 1.28. Additionally, YAN et al [21] also indicated that high temperature, low TiO2 content and high binary basicity of slag were beneficial to decreasing the vanadium distribution ratio between blast furnace slag and metal. NAN [25] proposed that high temperature and increased basicity were beneficial to the reduction of vanadium oxides in blast furnace hearth. The opposite conclusion concerning the impact of slag basicity in previous papers could be explained by strong oxygen potential conditions in the investigations of JUNG et al [16] and SHIN et al [17]. Moreover, previous researchers mainly focused on the vanadium behavior between the metal and ordinary slag or titanium slag with a low TiO2 content. The behavior of vanadium between molten iron and titanium slag with a high TiO2 content is still not clear.
In this study, the used vanadium titanomagnetite has a low silica and high titania and the addition of CaO and MgO were applied to adjusting slag composition. The effects of CaO content, MgO content and smelting temperature were analyzed thermodynamically and studied experimentally in an electric furnace. Moreover, the relationships among the slag optical basicity, CaO/MgO mole ratio and vanadium distribution ratio between the slag and the molten iron and vanadium recovery rate in pig iron were also discussed. These findings will support the development of the DR-EF process for the comprehensive utilization of vanadium titano- magnetite ore.
2 Experimental
2.1 Materials and methods
The raw material for producing vanadium titanomagnetite metalized pellets used in this study was taken from the Panxi region of China. The metallized pellet mainly contains 71.38 wt.% total iron, 15.53 wt.% TiO2, 0.82 wt.% V2O5 and 3.71 wt.% SiO2 [5]. All of chemicals and reagents utilized of this study are of analytical grade. The details of electric furnace and experimental steps have been described in our previous papers [5,8]. The metallized pellet powders were prepared by crusing the pellet and then mixed with additives (CaO, MgO) and graphite. The mixture was placed in a graphite crucible and smelted in the electric furnace at various temperatures under the protection of high purity argon gas. After the given smelting duration of 20 min, the sample was removed from the furnace and rapidly cooled under the protection of argon gas. At last, the vanadium-bearing pig iron and titanium slag were separated and crushed for subsequent analysis.
2.2 Definition of parameters
(1) Vanadium distribution ratio (LV)
The vanadium distribution ratio between the titanium slag and the molten iron is expressed as
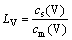 (1)
(1)
where cs(V) and cm(V) represent the contents of elemental vanadium in the titanium slag and the molten iron, respectively.
(2) Vanadium recovery rate (ηV)
The recovery rate of vanadium in pig iron is calculated by Eq. (2), and we also use the following expression to analyze the distributions of V between slag and iron:
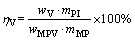 (2)
(2)
where wV and wMPV are the mass fractions of V in pig iron and vanadium-bearing titanomagnetite metallized pellets (wt.%), respectively; mPI and mMP are the masses of pig iron and vanadium-bearing titanomagnetite metallized pellets (g), respectively.
2.3 Analysis and characterization
FactSage 7.1 software [27] was adopted to predict the distribution behavior of vanadium during the smelting process. The “Equilib” module with the databases “FactPS” and “FToxid” was used. Besides, it should be mentioned that the value of the FactSage prediction might be not very accurate due to the insufficient thermodynamic data about vanadium oxides in its database. Nevertheless, the predicted trends may be correct and can be used to guide the experiments. The chemical composition of the titanium slag was determined by X-ray fluorescence (XRF) technique (PANalytical, The Netherlands). The vanadium content in the iron was determined by chemical analysis method.
7.1 software [27] was adopted to predict the distribution behavior of vanadium during the smelting process. The “Equilib” module with the databases “FactPS” and “FToxid” was used. Besides, it should be mentioned that the value of the FactSage prediction might be not very accurate due to the insufficient thermodynamic data about vanadium oxides in its database. Nevertheless, the predicted trends may be correct and can be used to guide the experiments. The chemical composition of the titanium slag was determined by X-ray fluorescence (XRF) technique (PANalytical, The Netherlands). The vanadium content in the iron was determined by chemical analysis method.
3 Thermodynamic analysis and calculations
In general, vanadium is in the form of vanadium spinel (FeO·V2O3) in the vanadium titanomagnetite concentrate, and its reduction processes by solid carbon are expressed as Reactions (3)-(5) [28]. The temperatures of these reactions are higher than 1339.1, 2008.1 and 1375 K, respectively. The metallized pellet was produced from the vanadium titanomagnetite concentrate pellet by the coal-based direct reduction in a rotary kiln at 1373 K with a C/Fe mole ratio of 1:1 for 3 h [5,8]. Therefore, it is clear that the vanadium oxides in the metallized pellets could be reduced to low valence vanadium oxides.
FeO·V2O3(s)+2C(s)=Fe(s)+2VO(s)+2CO(g),
ΔrGmΘ=426928-318.82T (3)
VO(s)+C(s)=V(s)+CO(g),
ΔrGmΘ=310493-154.62T (4)
VO(s)+C(s)=[V]+CO(g),
ΔrGmΘ=289768-210.64T (5)
(V2+)+(O2-)+C=[V]+CO (6)
According to Reaction (6), it can be inferred that the slag composition may influence the vanadium reduction and distribution. Thus, the effects of slag composition and smelting temperature on the vanadium distribution ratio between slag and metal were calculated and discussed as follows.
3.1 CaO addition
As we know, the low valence vanadium oxides (such as VO) are basic oxides in titanium slag during the smelting process. According to the ionic theory of molten slag [24], CaO is a strongly basic oxide which can donate oxygen ion (O2-) in molten slag. For the reduction of basic oxide, increasing slag basicity can promote the transformation of vanadium from slag to hot metal [24]. The effect of CaO content on the vanadium distribution ratio between the slag and the iron was analyzed by FactSage software with a constant MgO content of 10 wt.% in the titanium slag and smelting temperature of 1823 K. As shown in Fig. 1(a), the distribution ratio of vanadium (LV) between the slag and iron decreases with the increase of the CaO content in slag. This trend is similar to previous works [21,23].
software with a constant MgO content of 10 wt.% in the titanium slag and smelting temperature of 1823 K. As shown in Fig. 1(a), the distribution ratio of vanadium (LV) between the slag and iron decreases with the increase of the CaO content in slag. This trend is similar to previous works [21,23].
3.2 MgO addition
The effect of MgO content on the vanadium distribution ratio was calculated with a constant CaO/SiO2 mass ratio of 1.0 and smelting temperature of 1823 K. As shown in Fig. 1(b), the vanadium distribution ratio decreases with the increase of the MgO content in slag. Thus, similar to the influence of CaO, the vanadium distribution ratio decreases as the MgO content increases. The strength of basic oxide is related with the electrostatic potential of cations. The lower the electrostatic potential of cations is, the stronger the basicity of the corresponding oxide. The CaO is more basic than MgO due to the fact that the electrostatic potential of Ca2+ (1.89) is lower than that of Mg2+ (3.08) [28]. By comparing Fig. 1(a) with Fig. 1(b), the effect of MgO on the vanadium distribution is weaker than that of CaO because CaO is more basic than MgO.
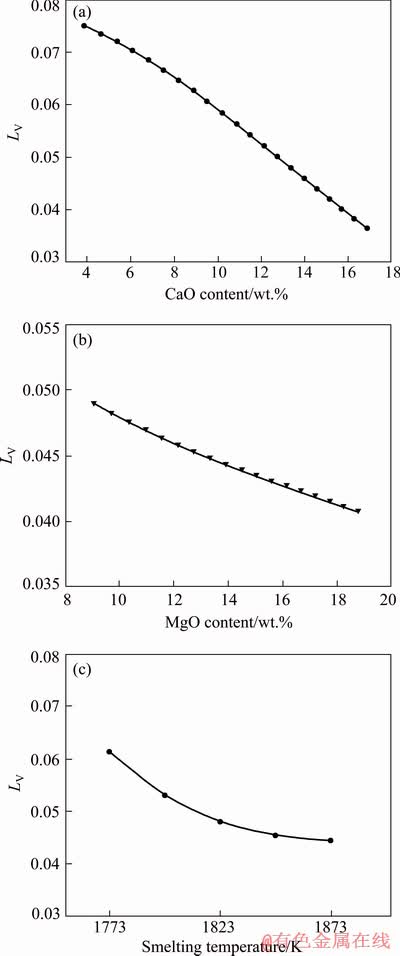
Fig. 1 Effects of CaO content (a), MgO content (b) and smelting temperature (c) on vanadium distribution ratio between slag and molten iron (predicted by FactSage 7.1)
3.3 Smelting temperature
Figure 1(c) shows the effect of smelting temperature on the distribution ratio of vanadium under a fixed slag composition. The vanadium distribution ratio decreases as the smelting temperature increases. This trend can be explained by the enhancement of endothermic reduction (Reaction (5)) of vanadium oxides with increasing the temperature.
4 Results and discussion
4.1 Effects of CaO content
High-temperature smelting experiments were conducted to investigate the effects of CaO content on the vanadium behavior during the smelting of vanadium titanomagnetite metallized pellets. A series of experiments were carried out at 1823 K for 20 min with 2 wt.% reductant. The experiments were carried out in a carbon crucible with graphite addition under high purity argon atmosphere resulting in a very low oxygen potential during the smelting process. As shown in Fig. 2(a), it can be seen that the content of vanadium in the hot iron tends to increase as the CaO content increases. Figure 2(b) shows that the distribution ratio of vanadium between the slag and the molten iron decreases with increasing the CaO content. As shown in Fig. 2(c), the recovery rate of vanadium in pig iron also increases with an increase in CaO content in slag. This trend is in agreement with the above thermodynamic analysis and previous investigations [21,23]. According to the above thermodynamic analysis, increasing CaO content can improve the reduction of vanadium oxides and promote the vanadium transfer from slag to molten iron. Besides, the increase of CaO content in slag can also reduce the viscosity and improve the fluidity of slag, therefore strengthening the dynamic condition of reduction reactions [29]. Thus, the increase of CaO content can improve the thermodynamic and dynamic conditions for the recovery of vanadium during the smelting process.
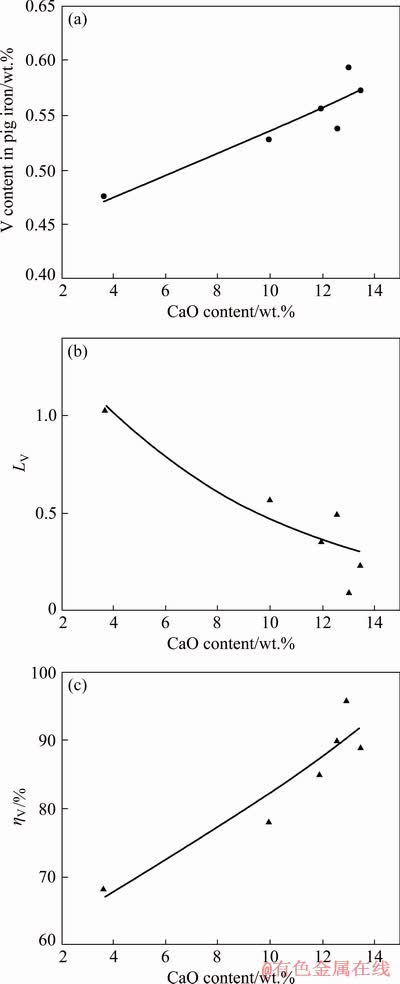
Fig. 2 Effects of CaO content in slag on vanadium content in pig iron (a), vanadium distribution ratio (b) and vanadium recovery rate in pig iron (c)
4.2 Effects of MgO content
The effects of MgO content on the vanadium behavior were investigated at the smelting temperature of 1823 K for 20 min with the reductant addition of 2 wt.% and constant CaO content. Figure 3(a) shows that the reduction of vanadium oxides is promoted and the vanadium content in the molten iron increases with the increasing MgO content in the slag. Figure 3(b) illustrates that the vanadium distribution ratio decreases as the MgO content increases. Furthermore, as shown in Fig. 3(c), the recovery rate of vanadium in pig iron has an increase tendency with increasing the MgO content in the slag. This trend is consistent with the thermodynamic analysis. The addition of MgO can improve the thermodynamic condition to improve the reduction of vanadium oxides during the smelting process. Moreover, similar with CaO, the increase of MgO in slag can also reduce slag viscosity [29] and improve the fluidity of slag so that promote the recovery of vanadium in pig iron. HOWARD et al [30] showed that the presence of MgO had no significant influence on vanadium distribution, which was attributed to high FeO content and too low content of MgO.
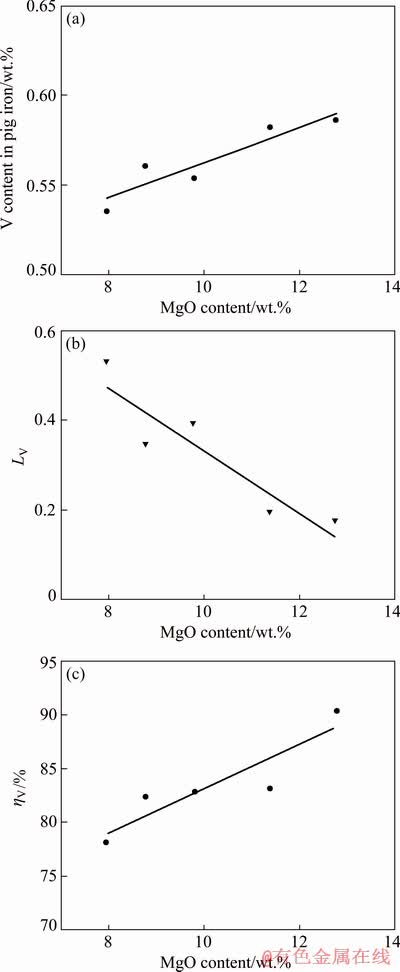
Fig. 3 Effects of MgO content in slag on vanadium content in pig iron (a), vanadium distribution ratio (b) and vanadium recovery rate in pig iron (c)
4.3 Effects of smelting temperature
The effects of smelting temperature on the vanadium behavior were studied at a fixed slag composition, 20 min duration and 2 wt.% reductant. Figures 4(a) and 4(b) show that the vanadium content in the iron increases as the smelting temperature increases, whereas the vanadium content decreases after the temperature is above 1848 K. The vanadium distribution ratio decreases when the smelting temperature increases from 1798 to 1848 K and it increases with the further increase of temperature. Figure 4(c) shows that the recovery rate of vanadium in pig iron increases when the smelting temperature increases from 1798 to 1848 K, whereas it decreases at higher smelting temperature. Previous investigations [16,17,21,26] indicated that the vanadium distribution ratio between slag and hot metal decreased as smelting temperature increased. It can be explained that increasing smelting temperature can improve the thermodynamic conditions of recovery of vanadium in pig iron. However, the higher smelting temperature will cause the over-reduction of titanium-bearing slag, which can deteriorate the fluidity of slag. This means the dynamic conditions probably are bad for the recovery of vanadium at higher temperature. Therefore, to achieve the higher recovery rate of vanadium in pig iron, reduce energy consumption and suppress the reduction of titanium oxides [8], the smelting temperature should be controlled to be not too high.
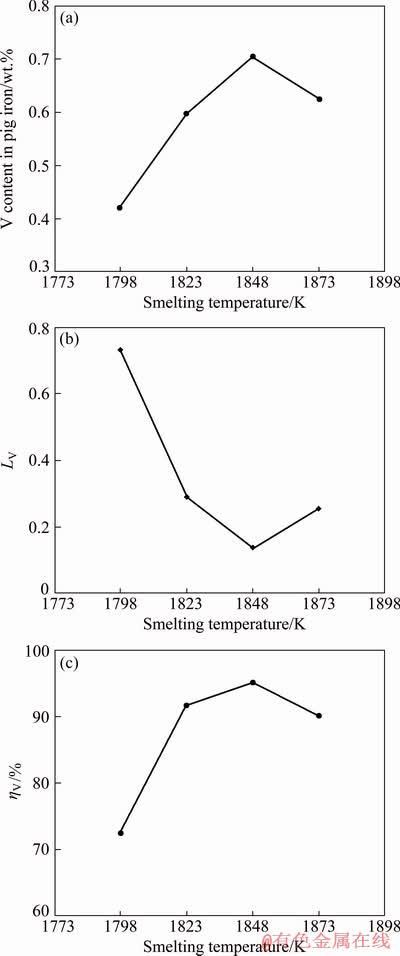
Fig. 4 Effects of smelting temperature on vanadium content in pig iron (a), vanadium distribution ratio (b) and vanadium recovery rate in pig iron (c)
4.4 Effects of optical basicity
The optical basicity of the oxides melt can be regarded as its tendency to liberate oxide ions and has a significant influence on the oxides reduction. Therefore, the relationship between the optical basicity of the slag and the V behavior was discussed to investigate the influence of slag composition. The relationship of the optical basicity of slag and the vanadium distribution ratio and vanadium recovery rate in pig iron was discussed as follows. The optical basicity [31] of the slag was calculated as
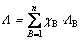 (7)
(7)
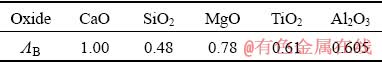
where ΛB represents the optical basicity of each oxide [28,31], and χB represents the mole fraction of positive ion in each oxide. The optical basicity and the mole fraction of each oxide in the slag are listed in Table 1 and Table 2, respectively. Additionally, previous studies [32,33] indicated that the vanadium oxide did not contribute to the optical basicity of the melts, and the vanadium oxide content in the slag is very tiny. Thus, the vanadium oxide was ignored in the calculation of the optical basicity of the slag in the present study.
Table 1 Optical basicity of each oxide
As illustrated in Fig. 5(a), the vanadium distribution ratio between the slag and the molten iron decreases as the optical basicity increases. Figure 5(b) shows that there is an increase tendency in the recovery rate of vanadium with the increase in the optical basicity of slag. It can be explained that the free oxygen ion (O2-) in the slag increases as the optical basicity increases, which is beneficial to the reduction of vanadium oxides from the thermodynamic view. Consistently, the previous studies [21,23] also showed that the increase of basicity could improve vanadium oxide reduction and decrease vanadium distribution ratio between titanium slag and metal. However, some investigators [14,18,26] reported that the increasing slag basicity suppressed the reduction of vanadium oxide and increased the vanadium distribution ratio between slag and metal. This difference might be as a result of a very high range of FeO used and high oxygen potential in their investigations. Under the high oxygen potential condition, the vanadium oxide would exist as high valence vanadium oxide (such as V2O5), which is an acidic oxide and could cause the opposite conclusion to this study (low valence vanadium oxide in slag under low oxygen potential) according to thermodynamic analysis. Furthermore, the increase of optical basicity of slag will increase the O2- ion amounts in molten slag, thus improving the fluidity of slag and the dynamic conditions of recovery of vanadium in pig iron.
Table 2 Mole fractions of each oxide in slag and corresponding optical basicity
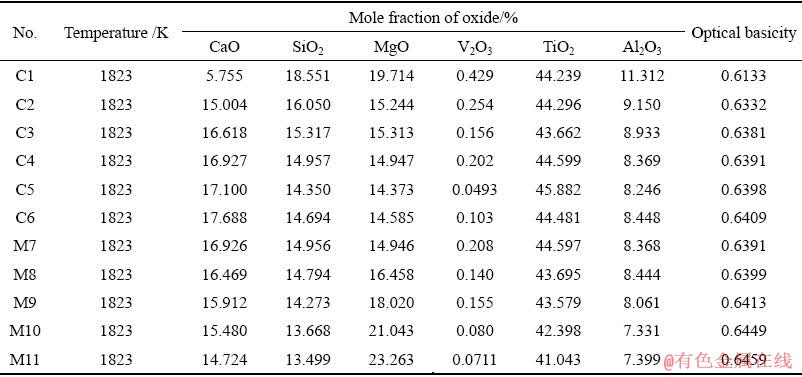
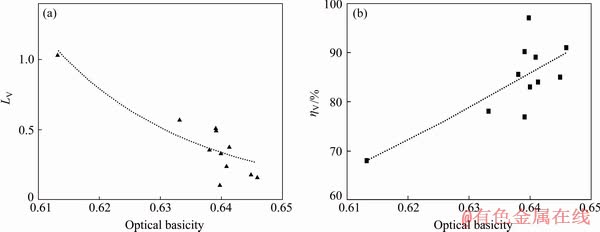
Fig. 5 Effect of optical basicity of slag on vanadium distribution ratio (a) and vanadium recovery rate in pig iron (b)
4.5 Effect of CaO/MgO mole ratio
The above results indicated that the increase of the slag optical basicity and the smelting temperature could decrease the vanadium distribution ratio between the slag and iron and increase the recovery rate of vanadium in pig iron. The vanadium reduction was improved by adding CaO and MgO in the slag, as well as increasing the smelting temperature. Furthermore, the extraction of titanium resource from the enriched titanium slag should be considered to achieve the comprehensive utilization of vanadium titanomagnetite. The main Ti-bearing phase is important for titanium extraction by acid leaching due to different acid solubilities of titanates. It is proven that increasing the proportion of MgTi2O5 phase in titanium slag results in a higher acid solubility ratio due to its good acid solubility [34], whereas CaTiO3 has a poor acid solubility [35]. Moreover, the increment of MgO content in titnaium slag is beneficial to MgTi2O5 formation and the CaO addition prefers to CaTiO3 formation. Reducing CaTiO3 proportion and increasing MgTi2O5 in slag is beneficial to titanium extraction by acid leaching. Therefore, the effects of the mole ratio of CaO/MgO on the vanadium distribution ratio and recovery rate of vanadium in pig iron are shown in Fig. 6.
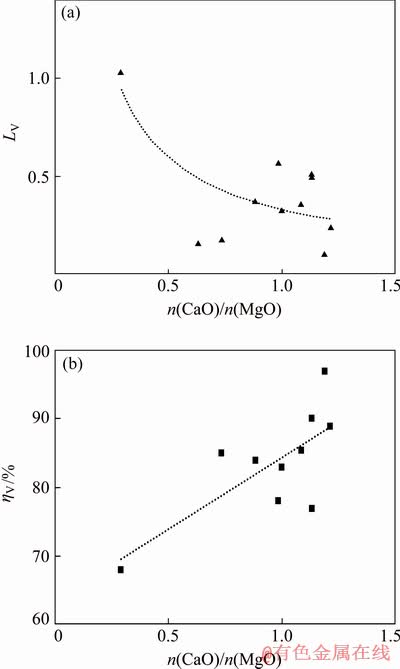
Fig. 6 Effects of CaO/MgO mole ratio in slag on vanadium distribution ratio (a) and vanadium recovery rate in pig iron (b)
As illustrated in Fig. 6, the vanadium distribution ratio decreases when the CaO/MgO mole ratio increases from about 0.4 to 0.8, and then it has insignificant changes with the further increase of the CaO/MgO mole ratio. This trend could be explained by the thermodynamic improvement of reduction of vanadium oxides with an increment of CaO/MgO mole ratio; on the other hand, the CaO/MgO additions simply improved the slag viscosity in this high titania slag and thereby made it easy for the reduction of vanadium oxides (Vox) and improved the dynamic conditions. Furthermore, with the limited addition of CaO, MgO can be added during the smelting process for the promotion of the vanadium reduction. In conclusion, both CaO and MgO are basic oxides, and adding MgO to increase the optical basicity of titanium slag can not only improve the recovery of vanadium in pig iron but also be beneficial to titanium extraction by acid leaching.
5 Conclusions
(1) The reduction and distribution behaviors of vanadium during the smelting of vanadium titanomagnetite metallized pellets were studied. The thermodynamics of additions of CaO and MgO and smelting temperature on the reduction and distribution of vanadium was analyzed and the various high temperature smelting experiments were conducted.
(2) The thermodynamic analysis indicated that increasing CaO content, MgO content or optical basicity in slag can improve the recovery of V in pig iron from the viewpoint of thermodynamics. The results of experiments showed that the additions of CaO and MgO in the high titania slag could improve the reduction of vanadium oxides and increase the recovery rate of vanadium in pig iron. The smelting temperature should not be higher than 1848 K to suppress the reduction of titanium oxides and achieve high recovery rate of vanadium in pig iron.
(3) The distribution ratio of vanadium between the high titania slag and the molten iron trended to decrease and the recovery rate of vanadium in pig iron trended to increase as the slag optical basicity increased. With the limited addition of CaO, adding MgO to increase the optical basicity of titanium slag could not only improve the recovery of vanadium but also be beneficial to titanium extraction by acid leaching.
References
[1] MOSKALYK R R, ALFANTAZI A M. Processing of vanadium: A review [J]. Minerals Engineering, 2003, 16(9): 793-805.
[2] FU Wei-guo, WEN Yong-cai, XIE Hong-en. Development of intensified technologies of vanadium-bearing titanomagnetite smelting [J]. Journal of Iron and Steel Research, International, 2011, 18(4): 7-10.
[3] ROHRMANN B. Vanadium in South Africa (metal review series No. 2) [J]. Journal of the Southern African Institute of Mining and Metallurgy, 1985, 85(5): 141-150.
[4] STEINBERG W, GEYSER W, NELL J. The history and development of the pyrometallurgical processes at Evraz Highveld Steel & Vanadium [J]. Journal of the Southern African Institute of Mining and Metallurgy, 2011, 111(10): 705-710.
[5] JIANG Tao, WANG Shuai, GUO Yu-feng, CHEN Feng, ZHENG Fu-qiang. Effects of basicity and MgO in slag on the behaviors of smelting vanadium titanomagnetite in the direct reduction-electric furnace process [J]. Metals, 2016, 6(5): 107.
[6] ZHANG Wen-sheng, ZHU Zhao-wu, CHENG Chu-yong. A literature review of titanium metallurgical processes [J]. Hydrometallurgy, 2011, 108(3-4): 177-188.
[7] ZHENG Fu-qiang, GUO Yu-feng, LIU Shui-shi, QIU Guan-zhou, CHEN Feng, JIANG Tao, WANG Shuai. Removal of magnesium and calcium from electric furnace titanium slag by H3PO4 oxidation roasting–leaching process [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(2): 356-366.
[8] WANG Shuai, GUO Yu-feng, JIANG Tao, CHEN Feng, ZHENG Fu-qiang, TANG Min-jun, YANG Ling-zhi, QIU Guan-zhou, Appropriate titanium slag composition during smelting of vanadium titanomagnetite metallized pellets [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(12): 2528-2537.
[9] TANG Wei-dong, YANG Song-tao, XUE Xiang-xin. Effect of B2O3 addition on oxidation induration and reduction swelling behavior of chromium-bearing vanadium titanomagnetite pellets with simulated coke oven gas [J]. Transactions of Nonferrous Metals Society of China, 2019, 29(7): 1549-1559.
[10] ZHENG Fu-qiang, CHEN Feng, GUO Yu-feng, JIANG Tao, TRAVYANOV A Y, QIU Guan-zhou. Kinetics of hydrochloric acid leaching of titanium from titanium-bearing electric furnace slag [J]. JOM, 2016, 68(5): 1476-1484.
[11] SONG Wen-chen, LI Hong, ZHU Fu-xing, LI Kun, ZHENG Quan, LI Hong. Extraction of vanadium from molten vanadium bearing slag by oxidation with pure oxygen in the presence of CaO [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(8): 2687-2694.
[12] PENG Xue-feng, ZHANG Yang, FAN Bing-qiang, ZHENG Shi-li, WANG Xiao-jian, ZHANG Ying, LI Ping, LIU Feng-qiang. Complexation separation for vanadium and chromium by dithiocarbamate and its application in treatment of chromium-vanadium-bearing slag [J]. Transactions of Nonferrous Metals Society of China, 2019, 29(11): 2400-2410.
[13] LI Wei, FU Gui-qin, CHU Man-sheng, ZHU Miao-yong. Influence of V2O5 content on the gas-based direct reduction of hongge vanadium titanomagnetite pellets with simulated shaft furnace gases [J]. JOM, 2018, 70(1): 76-80.
[14] INOUE R, SUITO H. Distribution of vanadium between liquid iron and magnesium oxide-saturated slags of the calcium oxide-magnesium oxide-iron oxides-silicon dioxide system [J]. Transaction Iron Steel Institute Japan, 1982, 22: 705-714.
[15] LIU Xuan, DIAO Jiang, KE Zhao-qun, QIAO Yong, ZHANG Tao, XIE Bing. Experimental investigation and thermodynamic modeling of vanadium, chromium and phosphorus distributions between FeO-SiO2-CaO-V2O3- Cr2O3-P2O5-MnO slag and semi-steel [J]. Metallurgical Research & Technology, 2016, 113(4): 407-419.
[16] JUNG W G, KIM H S, CHUNG S Y, CHOI H S. Equilibrium of vanadium between molten iron and CaO-SiO2- MgOsat.-FetO slag [J]. Steel GRIPS, 2003, 1: 361-366.
[17] SHIN D Y, WEE C H, KIM M S, YOU B D, HAN J W, CHOI S O, YUN D J. Distribution behavior of vanadium and phosphorus between slag and molten steel [J]. Metals and Materials International, 2007, 13(2): 171-176.
[18] DONG Jin-ming, ZHAO Fei, ZHANG Yan-ling, QIU Sheng-tao, GAN Yong. Distribution behaviors of vanadium between hot metal and FeO-SiO2-MnO(-TiO2) slag system and influential factors [J]. The Chinese Journal of Process Engineering, 2010, 10(6): 1076-1083. (in Chinese)
[19] ZHANG Y L, ZHAO F, WANG Y G. Effects of influencing factors on distribution behaviors of vanadium between hot metal and FeO-SiO2-MnO(-TiO2) slag system [J]. Steel Research International, 2011, 82(8): 940-950.
[20] WRIGHT S, DAVIDSON R. Distribution of vanadium between Al2O3-CaO-MgO-SiO2-TiO2 slag and carbon saturated iron [J]. Mineral Processing and Extractive Metallurgy, 2019. http://www.trandfonline.com.doi/full/10. 1080/25726641.2019.1633505.
[21] YAN Jia-rong, XIE Bing, ZENG Xiao-yi, HUANG Qing-yun, LI Hong-yi. Vanadium distribution between blast furnace slag and hot metal [C]//4th International Symposium on High-Temperature Metallurgical Processing. San Antonio, Texas, USA: TMS, 2013: 333-339.
[22] HUANG Shi-hong, LEI Ting, HAN Feng-xia, ZHOU Lin. Element distribution of titanium slag smelted by DC arc furnace [J]. Journal of Rare Metal, 2014, 38: 283-289. (in Chinese)
[23] WANG Zhen-yang, ZHANG Jian-liang, XING Xiang-dong, LIU Zheng-jian. Distribution behaviors and thermodynamic analysis of vanadium between molten iron and high titanium slag [J]. The Chinese Journal of Nonferrous Metals, 2015, 25: 1355-1361. (in Chinese)
[24] YANG Su-bo, LUO Ze-zhong, CAI Kai-ke, JIANG Jun-pu, SONG Bo. Thermodynamic study on distribution of vanadium between iron melt and slag in basic oxygen furnace [J]. Iron Steel, 2006, 41: 36-38. (in Chinese)
[25] NAN Xiang-min. Study on vanadium oxide reduction in blast furnace hearth [J]. Zhuzao Jishu, 2013, 34: 193-195. (in Chinese)
[26] LIU Tian-zhong, WANG Da-guang. Component distribution between carbon saturation molten iron and molten slag [J]. Iron Steel Vanadium Titanium, 1991, 12: 1-4. (in Chinese)
[27] BALE C W, BELISLE E, CHARTRAND P, DECTEROV S A, ERIKSSON G, GHERIBI A E, HACK K, JUNG I H, KANG Y B, MELANCON J, PELTON A D. FactSage thermochemical software and databases, 2010-2016 [J]. Calphad, 2016, 54: 35-53.
[28] HUANG Xi-hu. Iron and steel metallurgy principle [M]. 4th ed. Beijing: Metallurgical Industry Press, 2013. (in Chinese)
[29] ZHANG Sheng-fu, ZHANG Xi, LIU Wei, LV Xue-wei, BAI Chen-guang, WANG Long. Relationship between structure and viscosity of CaO-SiO2-Al2O3-MgO-TiO2 slag [J]. Journal of Non-Crystalline Solids, 2014, 402: 214-222.
[30] HOWARD R L, RICHARDS S S, WELCH B J, MOORE J J. Vanadium distribution in melts intermediate to ferroalloy production from vanadiferous slag [J]. Metallurgical and Materials Transactions B, 1994, 25(1): 27-32.
[31] GHOSH D, KRISHNAMURTHY V A, SANKARANARAYANAN S R. Application of optical basicity to viscosity of high alumina blast furnace slags [J]. Journal of Mining and Metallurgy, Section B: Metallurgy, 2010, 46(1): 41-49.
[32] FARAH H, BRUNGS M. Oxidation-reduction equilibria of vanadium in CaO-SiO2, CaO-Al2O3-SiO2 and CaO-MgO- SiO2 melts [J]. Journal of Materials Science, 2003, 38(9): 1885-1894.
[33] FARAH H. Optical basicity analysis of vanadium-bearing silicate glasses/melts [J]. Journal of the American Ceramic Society, 2008, 91(12): 3915-3919.
[34] KRAMER S M, GORICHEV I G, LAINER Y A, ARTAMONOVA I V, TEREKHOVA M V. Calculation of the solubility of TiO2 and titanates in sulfuric acid solutions [J]. Russian Metallurgy (Metally), 2015, 2014: 704-707.
[35] WU Min-zhi, LV Hui-hong, LIU Min-chao, ZHANG Zheng-li, WU Xing-rong, LIU Wei-ming, WANG Ping, LI Liao-sha. Direct extraction of perovskite CaTiO3 via efficient dissociation of silicates from synthetic Ti-bearing blast furnace slag [J]. Hydrometallurgy, 2017, 167: 8-15.
钒钛磁铁矿金属化球团还原冶炼中钒的行为
王 帅,郭宇峰,郑富强,陈 凤,杨凌志,姜 涛,邱冠周
中南大学 资源加工与生物工程学院,长沙 410083
摘 要:研究电炉冶炼钒钛磁铁矿金属化球团中炉渣氧化钙、氧化镁含量以及冶炼温度对钒的行为的影响。对钒的还原和分配热力学进行计算和分析,并采用实验室电炉进行高温冶炼试验。热力学计算结果表明,钒在钛渣和铁水间的分配比随着渣中氧化钙、氧化镁含量增加以及冶炼温度的提高而下降。电炉冶炼试验结果表明,铁水中的钒含量以及生铁中钒回收率随着渣中氧化钙、氧化镁含量以及冶炼温度的提高而增加,而钒在钛渣和铁水间的分配比则有下降的趋势。此外,钒在铁水中的回收率随着钛渣光学碱度的增加存在提高的趋势。增加钛渣中的氧化镁含量进而提高炉渣光学碱度,不仅有利于钒的还原,而且可以促进有利于钛回收的含镁黑钛石相的形成。
关键词:钒;钒钛磁铁矿;钒分配比;电炉钛渣;氧化镁
(Edited by Bing YANG)
Foundation item: Project (2019JJ50816) supported by the Natural Science Foundation of Hunan Province, China
Corresponding author: Yu-feng GUO; Tel:+86-731-88830346; E-mail: yfguo@csu.edu.cn
DOI: 10.1016/S1003-6326(20)65330-4
Abstract: The effects of CaO content, MgO content and smelting temperature on the vanadium behavior during the smelting of vanadium titanomagnetite metallized pellets were investigated. The thermodynamics of reduction and distribution of vanadium was analyzed and the high-temperature smelting experiments were carried out. The thermodynamic calculations show that the distribution ratio of vanadium between the slag and the hot metal decreases with the increments of CaO and MgO content in the slag as well as the increase of the smelting temperature. The smelting experiments demonstrate that the vanadium content in iron and the recovery rate of vanadium in pig iron increase as the CaO content, MgO content and smelting temperature increase, whereas the vanadium distribution ratio between the slag and iron tends to decrease. Moreover, the recovery rate of vanadium in pig iron has a rising trend with increasing the optical basicity of the slag. The addition of MgO in the slag to increase the slag optical basicity can not only improve the vanadium reduction but also promote the formation of magnesium-containing anosovite, which is beneficial to following titanium extraction.


