DOI: 10.11817/j.issn.1672-7207.2018.12.003
Al2O3基和AlxTi1-xN基复合涂层的电化学研究
周磊,张立,钟志强,陈宜,张华栋,罗国凯,肖桥平
(中南大学 粉末冶金国家重点实验室,湖南 长沙,410083)
摘 要:
曲线和电化学阻抗谱方法,研究化学气相沉积方法制备的TiN/TiCN/TiAlCNO/Al2O3(简称Al2O3基)复合涂层,阴极电弧离子镀方法制备的Al0.55Ti0.45N/TiN(简称Al0.55Ti0.45N基)和Al0.67Ti0.33N/TiN(简称Al0.67Ti0.33N基)复合涂层在3.5%NaCl(质量分数)溶液中的电化学腐蚀行为。研究结果表明:Al2O3基、Al0.67Ti0.33N基和Al0.55Ti0.45N基复合涂层的孔隙率依次为0.34%,0.33%和0.02%,对基体的保护率依次为99.63%~99.91%,99.75%~99.93%和99.96%~99.99%;涂层合金耐腐蚀性能之间的差异实质上是涂层之间的差异;涂层耐腐蚀性能从高到低为Al0.55Ti0.45N(总厚度3.8 μm),Al0.67Ti0.33N(总厚度6.3 μm),Al2O3(总厚度15.1 μm),其中总厚度最小的Al0.55Ti0.45N基涂层的耐腐蚀性能明显优于其他2种涂层的耐腐蚀性能;继续增大涂层厚度并不能进一步改善涂层对基体的保护效果和涂层的耐腐蚀性能。
关键词:
CVD复合涂层;PVD复合涂层;动电位极化曲线;电化学阻抗谱;涂层孔隙率;涂层保护率;
中图分类号:TG178 文献标志码:A 文章编号:1672-7207(2018)12-2915-08
Electrochemical study of Al2O3 based and AlxTi1-xN based composite coatings
ZHOU Lei, ZHANG Li, ZHONG Zhiqiang, CHEN Yi, ZHANG Huadong, LUO Guokai, XIAO Qiaoping
(State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China)
Abstract: Electrochemical corrosion behaviors of three composite coatings in 3.5% NaCl (mass fraction) solution were studied by potentiodynamic polarization curve and electrochemical impedance spectroscopy. The coatings include TiN/TiCN/TiAlCNO/Al2O3 (hereinafter referred to as Al2O3 based) prepared by chemical vapor deposition and Al0.55Ti0.45N/TiN (Al0.55Ti0.45N based) and Al0.67Ti0.33N/TiN (Al0.67Ti0.33N based) by cathode arc evaporation ion plating. The results show that for Al2O3 based, Al0.67Ti0.33N based and Al0.55Ti0.45N based composite coatings, the porosities are 0.34%, 0.33% and 0.02%, respectively; the coating protection efficiencies for the substrate are 99.63%-99.91%, 99.75%-99.93% and 99.96%-99.99%, respectively. The difference among the corrosion resistance of coated alloys is essentially caused by the kinds of coatings. The corrosion resistance of the coatings is ordered as: Al0.55Ti0.45N (total thickness 3.8 μm), Al0.67Ti0.33N (total thickness 6.3 μm), Al2O3 (total thickness 15.1 μm). Compared with the other two coatings substantially higher corrosion resistance of Al0.55Ti0.45N based composite coating with the smallest thickness is observed. The protective effect of the coating on the substrate and the corrosion resistance of the coating cannot be further improved by increasing the coating thickness.
Key words: CVD composite coating; PVD composite coating; potentiodynamic polarization curve; electrochemical impedance spectroscopy; coating porosity; coating protection efficiency
目前超过80%的车削刀片和大约70%的铣削刀片采用了涂层技术[1-2]。传统的TiN和TiAlN等单层硬质涂层切削刀具已难以满足现代制造业的需求,涂层结构逐渐向纳米化、多元多层化、复合化等方向发展。APERADOR等[3]采用磁控溅射技术在AISI 1045钢基体上沉积了[TiN/AlTiN]n纳米复合涂层,n分别为1,6,12和24,并对其耐腐蚀性能进行了相关研究。XIAO等[4]采用电弧离子镀技术在硬质合金基体上沉积了AlCrN/TiSiN复合涂层和AlTiN/TiSiN复合涂层,并对2种复合涂层的高温抗氧化性能进行了对比分析。多层化以其独特的成分与结构设计,可以有效提高涂层切削刀具的使用寿命和切削效率,并有效拓展其对服役工况的适用性[5]。面向高温、潮湿、高盐度等各种复杂服役工况,改善其服役性能是涂层硬质合金的重要研究方向[6]。涂层硬质合金的耐腐蚀性能是其特定服役工况条件下使用寿命的重要影响因素,其评价表征也就尤为重要[7]。目前,国内外对涂层的研究主要集中在涂层的材质、微观结构、制备工艺、抗高温氧化性和耐磨性等方面[8-12],对涂层在介质中的耐腐蚀性能研究较少,对涂层耐腐蚀性能的相关研究也主要围绕单层涂层来进行评价与分析[13-15],对复合涂层体系的电化学腐蚀行为研究报道较少。采用电化学方法不仅可以直接研究涂层的耐腐蚀性能,而且还可以对涂层孔隙率和涂层对基体的保护率进行评估。涂层孔隙率和涂层对基体的保护率是涂层的重要本征性能,与涂层的膜基结合力、涂层的耐磨性等密切相关。Al2O3基CVD复合涂层和AlxTi1-xN基PVD复合涂层是应用较为广泛的商业化复合涂层。本文作者以3种典型的商业硬质涂层为研究对象,研究涂层在3.5%(质量分数)NaCl溶液中的电化学腐蚀行为。
1 实验材料和方法
1.1 涂层制备与微观组织观察
采用合金晶粒度为1.0 μm的WC–10Co硬质合金作为涂层基体;样品直径为17 mm,高度为6 mm。采用Ionbond公司BPX–pro 530化学气相沉积(CVD)设备,在合金基体表面沉积Al2O3基复合涂层,涂层从内至外的成分依次为TiN,TiCN,TiAlCNO,Al2O3和TiN。因沉积温度较高,CVD涂层表面通常存在微裂纹。前期研究结果表明[16],湿喷砂+机械抛光处理可以有效愈合复合涂层表面的微裂纹。因此该CVD涂层后处理技术已经得到产业化应用。为了真实反映涂层刀片实际使用前的表面状态,对Al2O3基复合涂层进行了湿喷砂+机械抛光处理。
采用Oerlikon Metco公司基于阴极电弧离子镀技术的Metaplase物理气相沉积(PVD)设备,分别在合金基体表面沉积Al0.55Ti0.45N/TiN(简称Al0.55Ti0.45N基)和Al0.67Ti0.33N/TiN(简称Al0.67Ti0.33N基)复合涂层。首先在基体上沉积TiN涂层,接着沉积AlTiN/TiN纳米交替涂层,最后沉积AlTiN涂层。Al0.55Ti0.45N基和Al0.67Ti0.33N基复合涂层按照相同的表层厚度和相同的调制周期控制工艺进行沉积。
采用FEI Quanta FEG 250扫描电镜(SEM)观察复合涂层的表面形貌;采用ZEISS Supra55扫描电镜观察复合涂层原始态和研磨抛光腐蚀态断面形貌;采用能谱仪(EDS)对复合涂层表面微区成分进行分析。
1.2 电化学腐蚀实验
采用CHI660E电化学综合测试设备进行电化学腐蚀实验。腐蚀介质为涂层电化学研究中常用的3.5%(质量分数)的NaCl溶液[17],测试温度保持恒温((25±1) ℃)。采用三电极体系,其中饱和甘汞电极(SCE)作为参比电极,铂片电极作为辅助电极,工作电极为待测试样。为了消除表面状态对样品实际工作面积的影响,在测试前对样品进行表面轻度抛光处理。测试时将试样放置在一个工作面面积为1 cm2的聚四氟乙烯夹具中。为了保持扩散离子的稳定性,工作电极与铂电极之间的平行距离保持在1 cm左右,参比电极置于两者之间且靠近工作电极。进行电化学腐蚀实验前将样品浸泡60 min,以保证涂层表面状态的稳定性;随后进行开路电位曲线的测量,时间为30 min以保证获得稳定的开路电位。开路电位稳定后进行动电位极化曲线测量,电压测试范围为-0.8~0.8 V,电位扫描速度为0.5 mV/s。
开路电位稳定后进行开路电位下电化学阻抗谱(EIS)测量,测试频率范围为10-2~105 Hz,电压振幅为5 mV,自动灵敏度。采用ZSimpWin软件,选择相应的等效电路对EIS进行拟合,在此基础上计算EIS的相关参数。
2 结果与分析
2.1 涂层表面形貌
图1所示为3种复合涂层抛光态表面形貌和相应的微区成分分析结果。从图1可以看出:经过抛光处理后,3种涂层表面均比较平滑。在Al2O3基复合涂层表面观察到了一定数量的微孔,但未发现明显微裂纹,只检测到Al和O元素,表明TiN表层已经完全去除,湿喷砂+机械抛光处理能有效愈合涂层表面微裂纹。与Al0.55Ti0.45N基复合涂层相比,Al0.67Ti0.33N基复合涂层表面存在较多数量的液滴,经抛光后液滴脱落从而导致涂层表面存在明显的微孔现象;2种AlTiN基复合涂层表面均只检测到Al,Ti,N元素;尽管EDS对O和N元素分析的误差较大,但是这种误差对金属元素相对含量的分析影响较小,检测到的Al/Ti物质的量比分别为0.47/0.53和0.63/0.37,接近其原始成分。
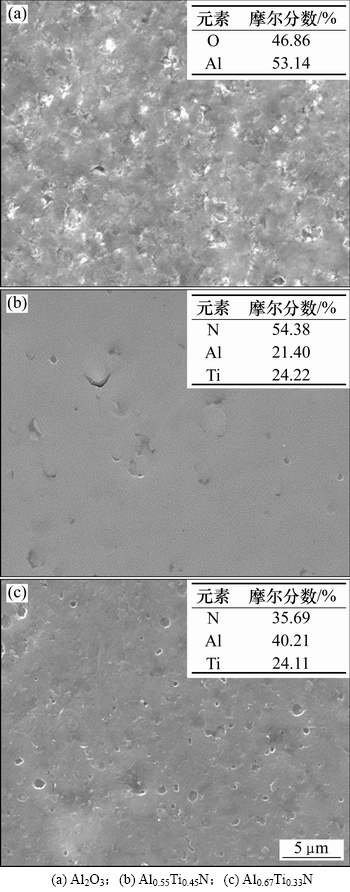
图1 Al2O3基、Al0.55Ti0.45N基和Al0.67Ti0.33N基复合涂层抛光态表面形貌的SEM照片和微区成分分析结果
Fig. 1 Surface SEM images and micro area composition analysis results of Al2O3 based, Al0.55Ti0.45N based and Al0.67Ti0.33N based composite coatings in a polished state
2.2 涂层断面形貌
3种复合涂层合金的原始态断面形貌分别见图2(a)~(c)。其中,图2(a)所示为涂层后处理态Al2O3基复合涂层,涂层从内至外依次为TiN,TiCN,TiAlCNO和Al2O3,涂层总厚度为15.1 μm;其中Al2O3层厚为4.6 μm,Al2O3和TiCN涂层均呈典型的柱状晶方式生长。由图2(b)和图2(c)可知:Al0.55Ti0.45N基和Al0.67Ti0.33N基复合涂层总厚度分别为3.8 μm和6.3 μm,均呈柱状晶方式生长;Al0.55Ti0.45N基复合涂层内部无明显缺陷,而Al0.67Ti0.33N基复合涂层内部存在熔滴、微孔等明显缺陷。2种复合涂层厚度不同的原因在于纳米交替层的数量差异。图2(d)和图2(e)所示为Al0.55Ti0.45N基复合涂层断面经抛光+腐蚀处理后的形貌,腐蚀液为HF+HNO3+H2O,腐蚀时间为30 s,表层Al0.55Ti0.45N涂层的厚度约为0.5 μm,Al0.55Ti0.45N/TiN纳米交替涂层中TiN涂层厚度约为18.3 nm,Al0.55Ti0.45N涂层厚度约为22.5 nm,调制周期约为40.8 nm。
2.3 动电位极化曲线和涂层孔隙率
基体和3种涂层合金的动电位极化数据以及涂层孔隙率如表1所示,其中,WC-10Co硬质合金基体、Al2O3基、Al0.55Ti0.45N基以及Al0.67Ti0.33N基复合涂层合金的动电位极化曲线见图3。Ecorr为自腐蚀电位,Jcorr为自腐蚀电流密度,Rp为极化电阻,βa为阳极极化率,βc为阴极极化率。
由表1可知:基体的自腐蚀电位为-0.54 V,负于3种复合涂层合金的自腐蚀电位。自腐蚀电位可表示材料失去电子的相对难易程度,电位越负表示材料腐蚀的热力学倾向越大[18]。这一结果表明,复合涂层硬质合金相对基体更不易发生腐蚀。自腐蚀电流密度是决定材料耐腐蚀性能的关键性判据,自腐蚀电流密度和材料腐蚀速度之间的对应关系可用式(1)表示[19]:
 (1)
(1)
式中:v为腐蚀速度,g·cm-2·h-1;M为材料的摩尔质量,g·mol-1;n为材料的原子价。由式(1)可知,材料的腐蚀速度与自腐蚀电流密度呈现正相关关系。
由表1可知:Al2O3基、Al0.67Ti0.33N基和Al0.55Ti0.45N基复合涂层合金的Jcorr依次为基体Jcorr的0.37%,0.25%和0.04%。由此可以推断,3种复合涂层均能有效阻止含氯介质对于基体的腐蚀,涂层合金耐腐蚀性能之间的差异实质上是涂层之间的差异,Al2O3基、Al0.67Ti0.33N基和Al0.55Ti0.45N基复合涂层合金的耐腐蚀性能依次增强。涂层成分、微观结构以及微孔等缺陷是涂层耐腐蚀性能差异的主要原因[20]。对成分属性相同或相近的涂层,涂层孔隙缺陷是影响涂层耐腐蚀性能的主要因素[21–22]。涂层的孔隙率可由式(2)计算[23-24]:
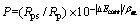 (2)
(2)
式中:P为涂层孔隙率,%;Rps为基体的极化电阻,Ω·cm2;Rp为涂层的极化电阻,Ω·cm2;△Ecorr为涂层合金与基体自腐蚀电位的差值,V。由动电位极化曲线计算所得的Al2O3基、Al0.67Ti0.33N基和Al0.55Ti0.45N基复合涂层的孔隙率分别为0.34%,0.33%和0.02%,这一分析结果与对图1的定性分析结果以及涂层的耐腐蚀性能具有较好的一致性。易于在涂层表面形成液滴缺陷是阴极电弧离子镀技术的本征属性。DING等[25]的研究结果表明,由于Al熔点较低,高Al含量的靶材在阴极电弧蒸发过程中更容易使涂层表面形成明显的液滴,导致涂层粗糙度和表面缺陷增多。结合涂层孔隙率分析结果可知:Al0.55Ti0.45N基复合涂层良好的表面状态和低的涂层孔隙率是其耐腐蚀性能明显优于其他2种涂层的主要原因。
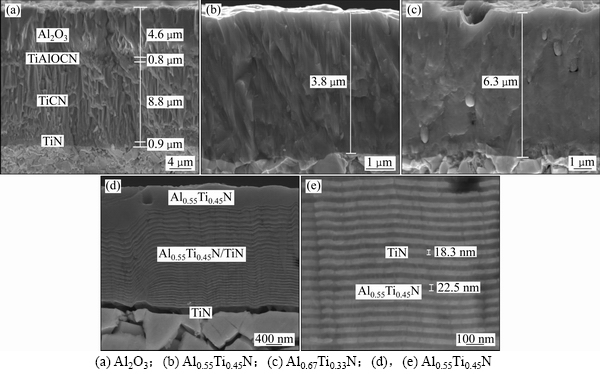
图2 Al2O3基、Al0.55Ti0.45N基和Al0.67Ti0.33N基复合涂层合金断面形貌的SEM照片
Fig. 2 Fracture surface SEM images of Al2O3 based, Al0.55Ti0.45N based and Al0.67Ti0.33N based composite films coated alloys
表1 基体和3种涂层合金的动电位极化数据以及涂层孔隙率
Table 1 Potentiodynamic polarization data of substrate and three coated alloys and corresponding coating porosities
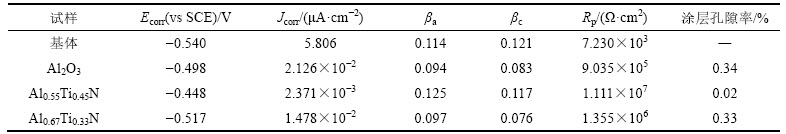
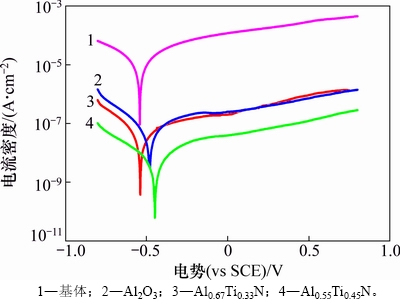
图3 基体和3种涂层合金的动电位极化曲线
Fig. 3 Potentiodynamic polarization curves of substrate and three coated alloys
2.4 电化学阻抗图谱分析
WC-10Co硬质合金基体、Al2O3基、Al0.55Ti0.45N基以及Al0.67Ti0.33N基复合涂层合金的Nyquist阻抗图见图4。结果表明,腐蚀介质若渗透至涂层内部界面处并发生腐蚀反应,电化学阻抗图谱会出现第2个时间常数[26]。由图4可知:3种复合涂层的Nyquist图均表现为一个明显的容抗弧,只存在一个时间常数,表明腐蚀介质还未明显渗透至复合涂层的次表层,未发生明显的层间腐蚀。这一现象表明,在此实验条件下,涂层对于腐蚀介质的渗透具有很强的阻挡能力。这一分析结果与动电位极化曲线测试结果具有很好的一 致性。
图5所示为基体与AlxTi1-x的电化学腐蚀等效电路模型,图5中Rs为工作电极与参比电极之间的溶液电阻,Qcoat为涂层电容,Rcoat为涂层电阻,Rct为电荷转移电阻,Qdl为双电层电容。基体与腐蚀介质界面间的腐蚀反应由并联的Qdl和Rct表征。Al2O3基和AlxTi1-xN基复合涂层的电化学腐蚀等效电路模型一致。复合涂层与腐蚀介质界面间的腐蚀反应由并联的Rcoat和Qcoat表征,腐蚀介质通过孔隙和缺陷渗透进入涂层内部发生的腐蚀反应由Qdl和Rct表征。
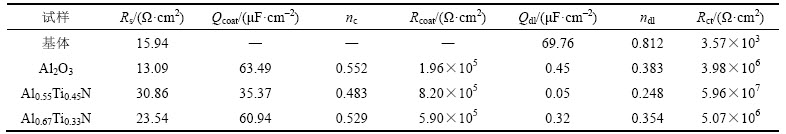
采用ZSimpWin软件对基体和3种复合涂层对应的等效电路进行拟合,得到的相关EIS电化学参数如表2所示。电化学腐蚀过程中固体电极电容的频响特性与纯电容不一致,存在一定的偏离,因此在等效电路中电容均采用常相位角元件Q来描述,以消除非理想电容的影响。表2中的nc和ndl分别为Qcoat和Qdl的偏离参数,参数n的变化范围为0<n<1。当n=0时,Q还原为R(电阻);当n=1时,Q变为C(电容)[27]。在平衡电位的EIS图谱中,涂层阻挡腐蚀介质渗透的能力与Rcoat和Qcoat的相关关系分别为正相关和负相 关[28]。由表2可知:Al0.67Ti0.33N基和Al0.55Ti0.45N基复合涂层的Rcoat分别为Al2O3基复合涂层Rcoat的3.0倍和4.2倍;Al2O3基和Al0.67Ti0.33N基复合涂层的Qcoat分别为Al0.55Ti0.45N基复合涂层Qcoat的1.8倍和1.7倍,表明3种涂层中Al0.55Ti0.45N基复合涂层阻挡腐蚀介质渗透的能力最强,即耐腐蚀性能最优。容抗弧的半径是电化学阻抗谱中反映材料耐腐蚀性能的直观判据,容抗弧半径越大,材料的耐腐蚀性能越好[7],而容抗弧半径与Rct和Qdl的相关关系分别为正相关和负相关。由表2可知,Al2O3基、Al0.67Ti0.33N基和Al0.55Ti0.45N基复合涂层的Rct分别为基体Rct的1 115倍、1 420倍和16 695倍;基体的Qdl分别为Al2O3基、Al0.67Ti0.33N基和Al0.55Ti0.45N基复合涂层Qdl的155倍、218倍和1 395倍。以上电化学参数结果均表明3种复合涂层耐腐蚀性能从高到低依次为Al0.55Ti0.45N,Al0.67Ti0.33N,Al2O3,其中Al0.55Ti0.45N基涂层的耐腐蚀性能明显比其他2种涂层的高。
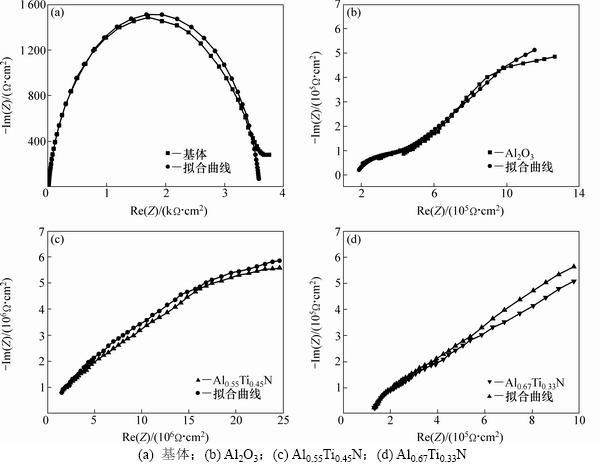
图4 基体和3种复合涂层合金的Nyquist阻抗图
Fig. 4 Nyquist impedance diagrams of substrate and three coated alloys
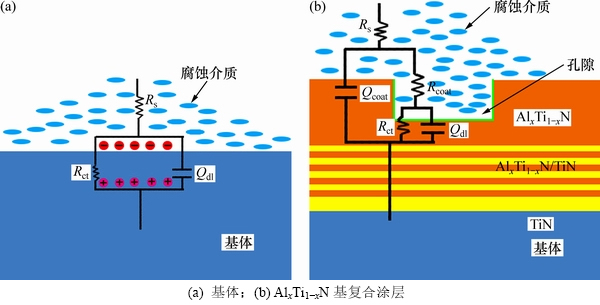
图5 基体和AlxTi1-xN基复合涂层合金的电化学腐蚀等效电路模型
Fig. 5 Electrochemical corrosion equivalent circuit models for substrate and AlxTi1-xN based composite films coated alloys
表2 拟合基体和3种复合涂层合金的等效电路得到的EIS电化学参数
Table 2 EIS electrochemical parameters obtained by equivalent circuit for substrate and three coated alloys
2.5 涂层保护率
在涂层电化学腐蚀研究中,可通过Pi或Pk来表征涂层对基体的保护程度,分别由式(3)和式(4)[29-31]计算得到:
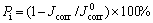 (3)
(3)
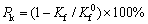 (4)
(4)
式中:J0 corr为基体的自腐蚀电流密度,μA·cm-2;Kf和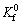 分别为涂层和基体Rct的倒数,Ω-1·cm-2。
分别为涂层和基体Rct的倒数,Ω-1·cm-2。
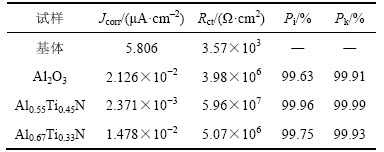
表3所示为3种复合涂层对基体保护率的相关数据。由表3可知:Al2O3基、Al0.67Ti0.33N基和Al0.55Ti0.45N 基复合涂层对基体的保护率依次增强,依次为99.63%~99.91%,99.75%~99.93%和99.96%~99.99%,表明在本实验条件下3种复合涂层均可有效保护基体合金免受腐蚀介质的侵蚀;同时可以看出,与动电位极化曲线分析方法相比,电化学阻抗图谱分析方法得出的涂层对基体的保护率分析结果稍高,提高幅度为0.03%~0.28%,在分析误差范围之内。
表3 Al2O3基、Al0.55Ti0.45N基和Al0.67Ti0.33N基复合涂层对基体的保护率(Pi和Pk)
Table 3 Protection rate (Pi and PK) of Al2O3 based, Al0.55Ti0.45N based and Al0.67Ti0.33N based composite coatings to substrate
3 结论
1) Al2O3基、Al0.67Ti0.33N基和Al0.55Ti0.45N基复合涂层合金的自腐蚀电流密度Jcorr分别为基体Jcorr的0.37%,0.25%和0.04%,电荷转移电阻Rct分别为基体Rct的1 115倍、1 420倍和16 695倍;Al2O3基、Al0.67Ti0.33N基和Al0.55Ti0.45N基复合涂层的孔隙率分别为0.34%,0.33%和0.02%,对基体的保护率分别为99.63%~99.91%,99.75%~99.93%和99.96%~99.99%。涂层合金耐腐蚀性能之间的差异实质上是涂层之间的差异。
2) 3种复合涂层合金均未发生明显的层间腐蚀,复合涂层均能有效阻止含氯溶液对基体的腐蚀;涂层耐腐蚀性能从高到低依次为Al0.55Ti0.45N(总厚度3.8 μm),Al0.67Ti0.33N(总厚度6.3 μm),Al2O3(总厚度15.1 μm),其中总厚度最小的Al0.55Ti0.45N基涂层的耐腐蚀性能明显优于其他2种涂层的耐腐蚀性能;继续增大涂层厚度并不能进一步改善涂层对基体的保护效果和涂层的耐腐蚀性能。
3) 电化学方法不仅可以直接研究涂层的耐腐蚀性能,同时也是一种表征涂层本征缺陷的有效方法。
参考文献:
[1] HAUBNER R, LESSIAK M, PITONAK R, et al. Evolution of conventional hard coatings for its use on cutting tools[J]. International Journal of Refractory Metals and Hard Materials, 2017, 62: 210-218.
[2] ORTNER H M, ETTMAYER P, KOLASKA H, et al. The history of the technological progress of hardmetals[J]. International Journal of Refractory Metals and Hard Materials, 2014, 44: 148-159.
[3] APERADOR W, CAICEDO J C, CABRERA G, et al. Bilayer period effect on corrosion-erosion resistance for [TiN/AlTiN]n multilayered growth on AISI 1045 steel[J]. Journal of Physics and Chemistry of Solids, 2010, 71(12): 1754-1759.
[4] XIAO Baijun, LI Haixu, MEI Haijuan, et al. A study of oxidation behavior of AlTiN-and AlCrN-based multilayer coatings[J]. Surface and Coatings Technology, 2018, 333: 229-237.
[5] SHAN Lei, WANG Yongxin, LI Jinlong, et al. Structure and mechanical properties of thick Cr/Cr2N/CrN multilayer coating deposited by multi-arc ion plating[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(4): 1135-1143.
[6] 冯于平. AlN基硬质涂层热稳定性、高温氧化行为及耐腐蚀性能研究[D]. 长沙: 中南大学粉末冶金研究院, 2014: 11-12.
FENG Yuping. Thermal stability, oxidation behavior and corrosion properties of AlN-based hard coating[D]. Changsha: Central South University. Powder Metallurgy Research Institute, 2014: 11-12.
[7] ZHANG Li, CHEN Yi, FENG Yuping, et al. Electrochemical characterization of AlTiN, AlCrN and AlCrSiWN coatings[J]. International Journal of Refractory Metals and Hard Materials, 2015, 53: 68-73.
[8] 牛瑞丽, 李金龙, 刘栓, 等. 偏压对高速钢表面AlTiN涂层结构与性能的影响[J]. 中国有色金属学报, 2016, 26(12): 2564-2571.
NIU Ruili, LI Jinlong, LIU Shuan, et al. Effect of bias on structure and properties of AlTiN coating deposited on high-speed steel[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(12): 2564-2571.
[9] CHANG Yinyu, WENG Shiyao, FU Fuxing, et al. High temperature oxidation and cutting performance of AlCrN, TiVN and multilayered AlCrN/TiVN hard coatings[J]. Surface and Coatings Technology, 2017, 332: 494-503.
[10] 张雨萌, 朱丽慧, 倪旺阳, 等. Al含量对TiAlN涂层热稳定性能的影响[J]. 中南大学学报(自然科学版), 2013, 44(7): 2696-2701.
ZHANG Yumeng, ZHU Lihui, NI Wangyang, et al. Effect of Al content on thermal stability of TiAlN coatings[J]. Journal of Central South University (Science and Technology), 2013, 44(7): 2696-2701.
[11] WANG Yanfeng, LI Zhengxian, WANG Haonan, et al. Effect of multilayered structure on properties of Ti/TiN coating[J]. Rare Metal Materials and Engineering, 2017, 46(5): 1219-1224.
[12] 王进春, 孔德军. 超音速火焰喷涂WC–12Co涂层高温摩擦–磨损性能[J].中南大学学报(自然科学版), 2017, 48(3): 608-616.
WANG Jinchun, KONG Dejun. Friction-wear properties of HVOF sprayed WC–12Co coatings at high temperatures[J]. Journal of Central South University (Science and Technology), 2017, 48(3): 608-616.
[13] MATEI A A, PENCEA I, BRANZEI M, et al. Corrosion resistance appraisal of TiN, TiCN and TiAlN coatings deposited by CAE-PVD method on WC–Co cutting tools exposed to artificial sea water[J]. Applied Surface Science, 2015, 358: 572-578.
[14] SHAN Lei, ZHANG Yangrong, WANG Yongxin, et al. Corrosion and wear behaviors of PVD CrN and CrSiN coatings in seawater[J]. Transactions of Nonferrous Metals Society of China, 2016, 26(1): 175-184.
[15] CHEN Mohan, CAI Fei, CHEN Wanglin, et al. Influence of vacuum annealing on structure and properties of AlTiSiN coatings with corrosion resistance[J]. Surface and Coatings Technology, 2017, 312: 25-31.
[16] ZHONG Zhiqiang, ZHANG Li, ZHOU Lei, et al. Cutting performances and the related characteristics of CVD coated hardmetal inserts changed by post-treatments[J]. International Journal of Refractory Metals and Hard Materials, 2018, 70: 162-168.
[17] FATEME A, HADI S. Surface nanostructure modification of Al substrate by N+ ion implantation and their corrosion inhibition[J]. Transactions of Nonferrous Metals Socienty of China, 2017, 27(3): 701-710.
[18] 陈蕾, 张维丹, 张媛媛. 氯离子对2种牙科常用合金耐腐蚀性的影响[J]. 中南大学学报(医学版), 2014, 39(11): 1186-1190.
CHEN Lei, ZHANG Weidan, ZHANG Yuanyuan. Effect of chlorde ion on corrosion of two commonly used dental alloys[J]. Journal of Central South University (Medical Science), 2014, 39(11): 1186-1190.
[19] 王凤平, 康万利, 敬和民, 等. 腐蚀电化学原理、方法及应用[M]. 北京: 化学工业出版社, 2008: 13-14.
WANG Fengping, KANG Wanli, JIN Hemin, et al. Principles, methods and applications of corrosion electrochemistry[M]. Beijing: Chemical Industry Press, 2008: 13-14.
[20] HASAN E, FARIDREZA A, ARASH F, et al. Microstructural and electrochemical comparision between TiN coatings deposited through HIPIMS and DCMS techniques[J]. Journal of Alloys and Compounds, 2018, 735: 422-429.
[21] 陈庆军, 将薇, 高霁雯. 涂层与块体致密度差值对铁基非晶合金耐蚀性的影响[J]. 中国有色金属学报, 2015, 25(9): 2414-2420.
CHEN Qingjun, JIANG Wei, GAO Jiwen. Effect of relative density difference between coating and bulk on Fe-based amorphous alloys corrosion resistance[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(9): 2414-2420.
[22] BARR S T, TRIBOLLET B, STEPHAN O, et al. Characterization of the porosity of silicon nitride thin layers by electrochemical impedance spectroscopy[J]. Electrochimica Acta, 2017, 227: 1-6.
S T, TRIBOLLET B, STEPHAN O, et al. Characterization of the porosity of silicon nitride thin layers by electrochemical impedance spectroscopy[J]. Electrochimica Acta, 2017, 227: 1-6.
[23] MATTHES B, BORSZEIT E, AROMA J, et al. Corrosion performance of some titanium-based hard coatings[J]. Surface and Coatings Technology, 1991, 49(1/2/3): 489-495.
[24] MENDIZABAL L, BAY N R, BARRIGA J, et al. Effect of N2 flow rate on the microstructure and electrochemical behavior of TaNx films deposited by modulated pulsed power magnetron sputtering[J]. Thin Solid Films, 2016, 610: 1-9.
N R, BARRIGA J, et al. Effect of N2 flow rate on the microstructure and electrochemical behavior of TaNx films deposited by modulated pulsed power magnetron sputtering[J]. Thin Solid Films, 2016, 610: 1-9.
[25] DING Xingzhao, TAN A L K, ZENG X T, et al. Corrosion resistance of CrAlN and TiAlN coatings deposited by lateral rotating cathode arc[J]. Thin Solid Films, 2008, 516(16): 5716-5720.
[26] JIANG Qiong, MIAO Qiang, TONG Fei, et al. Electrochemical corrosion behavior of arc sprayed AL-Zn-Si-RE coatings on mild steel in 3.5% NaCl solution[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(8): 2713-2722.
[27] 张鉴清, 曹楚南. 电化学阻抗谱方法研究评价有机涂层[J]. 腐蚀与防护, 1998, 19(3): 99-104.
ZHANG Jianqing, CAO Chunan. Study and evaluation on coatings by electrochemical impedance spectroscopy[J]. Corrosion and Protection, 1998, 19(3): 99-104.
[28] 曾佳俊, 郑鹏华, 邓尼丝, 等. 复合涂层体系浸泡失效过程电化学阻抗谱特征[J]. 腐蚀科学与防护技术, 2016, 28(1): 33-38.
ZENG Jiajun, ZHENG Penghua, DENG Nisi, et al. The electrochemical impedance spectrum characteristics of the composite coating system were investigated[J]. Corrosion Science and Protection Technology, 2016, 28(1): 33-38.
[29] JARMO L, PERTTU S, MIKAEL B, et al. Corrosion protection of steel with multilayer coatings: Improving the sealing properties of physical vapor deposition CrN coatings with Al2O3/TiO2 atomic layer deposition nanolaminates[J]. Thin Solid Films, 2017, 627: 59-68.
[30] SAJJAD G, ALI S, CHU P K. Corrosion behavior of reactive sputtered Ti/TiN nanostructure coating and effects of intermediate titanium layer on self-healing properties[J]. Surface and Coatings Technology, 2017, 326: 156-164.
[31] NOZAWA K, ARAMAKI K. One-and two-dimensional polymer films of modified alkanethiol monolayers for preventing iron from corrosion[J]. Corrosion Science, 1999, 41(1): 57-73.
(编辑 赵俊)
收稿日期:2017-12-12;修回日期:2018-03-17
基金项目(Foundation item):国家自然科学基金资助项目(51574292);中南大学贵重仪器设备开放共享基金资助项目(2017gxjj014);粉末冶金国家重点实验室基金资助项目(2017zzkt21)(Project(51574292) supported by the National Natural Science Foundation of China; Project(2017gxjj014) supported by the Open-End Fund for the Valuable and Precision Instruments of Central South University; Project(2017zzkt21) supported by the Foundation of the State Key Laboratory of Powder Metallurgy)
通信作者:张立,博士,教授,从事硬质合金材料科学与工程研究;E-mail:zhangli@csu.edu.cn
摘要:采用动电位极化曲线和电化学阻抗谱方法,研究化学气相沉积方法制备的TiN/TiCN/TiAlCNO/Al2O3(简称Al2O3基)复合涂层,阴极电弧离子镀方法制备的Al0.55Ti0.45N/TiN(简称Al0.55Ti0.45N基)和Al0.67Ti0.33N/TiN(简称Al0.67Ti0.33N基)复合涂层在3.5%NaCl(质量分数)溶液中的电化学腐蚀行为。研究结果表明:Al2O3基、Al0.67Ti0.33N基和Al0.55Ti0.45N基复合涂层的孔隙率依次为0.34%,0.33%和0.02%,对基体的保护率依次为99.63%~99.91%,99.75%~99.93%和99.96%~99.99%;涂层合金耐腐蚀性能之间的差异实质上是涂层之间的差异;涂层耐腐蚀性能从高到低为Al0.55Ti0.45N(总厚度3.8 μm),Al0.67Ti0.33N(总厚度6.3 μm),Al2O3(总厚度15.1 μm),其中总厚度最小的Al0.55Ti0.45N基涂层的耐腐蚀性能明显优于其他2种涂层的耐腐蚀性能;继续增大涂层厚度并不能进一步改善涂层对基体的保护效果和涂层的耐腐蚀性能。
[6] 冯于平. AlN基硬质涂层热稳定性、高温氧化行为及耐腐蚀性能研究[D]. 长沙: 中南大学粉末冶金研究院, 2014: 11-12.
[8] 牛瑞丽, 李金龙, 刘栓, 等. 偏压对高速钢表面AlTiN涂层结构与性能的影响[J]. 中国有色金属学报, 2016, 26(12): 2564-2571.
[10] 张雨萌, 朱丽慧, 倪旺阳, 等. Al含量对TiAlN涂层热稳定性能的影响[J]. 中南大学学报(自然科学版), 2013, 44(7): 2696-2701.
[12] 王进春, 孔德军. 超音速火焰喷涂WC–12Co涂层高温摩擦–磨损性能[J].中南大学学报(自然科学版), 2017, 48(3): 608-616.
[18] 陈蕾, 张维丹, 张媛媛. 氯离子对2种牙科常用合金耐腐蚀性的影响[J]. 中南大学学报(医学版), 2014, 39(11): 1186-1190.
[19] 王凤平, 康万利, 敬和民, 等. 腐蚀电化学原理、方法及应用[M]. 北京: 化学工业出版社, 2008: 13-14.
[21] 陈庆军, 将薇, 高霁雯. 涂层与块体致密度差值对铁基非晶合金耐蚀性的影响[J]. 中国有色金属学报, 2015, 25(9): 2414-2420.
[27] 张鉴清, 曹楚南. 电化学阻抗谱方法研究评价有机涂层[J]. 腐蚀与防护, 1998, 19(3): 99-104.
[28] 曾佳俊, 郑鹏华, 邓尼丝, 等. 复合涂层体系浸泡失效过程电化学阻抗谱特征[J]. 腐蚀科学与防护技术, 2016, 28(1): 33-38.


