Cu-In体系的热力学优化
北京有色金属研究总院矿物资源与冶金材料研究所,北京有色金属研究总院矿物资源与冶金材料研究所,北京有色金属研究总院矿物资源与冶金材料研究所,北京有色金属研究总院矿物资源与冶金材料研究所,北京有色金属研究总院矿物资源与冶金材料研究所 北京100088,北京100088,北京100088,北京100088,北京100088
摘 要:
评估了Cu-In体系的相平衡和热力学实验结果, 并对体系进行了热力学优化。采用替代模型描述体系的液相和富铜固溶体相, 双亚晶格模型描述线性化合物相, 三亚晶格模型描述非线性化合物相, 结合选取合理的实验数据, 优化得到Cu-In体系各相的热力学参数, 用优化结果计算的相图以及热力学性质与实验结果吻合。
关键词:
中图分类号: TG111.3
作者简介:储茂友 (E-mail:chumaoyou@163.com) ;
收稿日期:2006-12-25
基金:国家自然科学基金资助项目 (50672012);
Thermodynamic Optimization of Cu-In System
Abstract:
The data of phase diagram and thermodynamic properties of the Cu-In system in the literature were reviewed.The substitutional model was used for describing liquid and (Cu) terminal solid solution phases and two-sub lattice model for linear compounds and three-sub lattice model for non-stoichiometric compounds, respectively.Based on the available experimental data the thermodynamic parameters were optimized.The calculated phase diagram and thermodynamic properties were in reasonable agreement with experimental data.
Keyword:
Cu-In;phase diagram calculation;thermodynamic optimization;
Received: 2006-12-25
建立Cu-In体系的热力学模型, 对开发包含Cu, In元素的无铅焊料、 铜铟硒基太阳能电池材料有重要意义。 本文综合评估了Cu-In体系的相平衡和热力学实验结果, 选择适当的热力学模型, 进行热力学优化, 得到此二元系中各相的热力学参数。
1 实 验
Subramanian等
Kang等
Kao等
2 热力学模型
2.1 纯组元
对体系中的每一相φ, 其纯组元的摩尔自由焓°G
°G
其中, HiSER是元素i在298.15 K标准参考态 (Standard Element Reference -SER) 下的摩尔焓, a, b, c……为温度系数。 Cu和In各相纯组元的参数见表1, 取自SGTE热力学数据库
表1 纯元素热力学参数*
Table 1 Unary data for pure components
| Phase | Temperature range/K |
a | b | c | d | e | f | g | h | i |
Liquid Cu |
298.15~1358 | 5194.277 | 120.973331 | -24.112392 | -2.65684×10-3 | -1.29223×10-7 | 0 | -5.849×10-21 | 0 | 52478 |
| 1358~3200 | -46.545 | 173.881484 | -31.38 | 0 | 0 | 0 | 0 | 0 | 0 | |
Liquid In |
298.15~494.4 | -3696.798 | 84.701255 | -21.8386 | -5.72566×10-3 | -2.12032×10-6 | 0 | -5.59×10-20 | 0 | -22906 |
| 494.4~3800 | 3749.81 | 116.835784 | -27.4562 | 0.54607×10-3 | -0.08367×10-6 | 0 | 0 | 0 | -211708 | |
Fcc Cu |
298.15~1358 | -7770.458 | 130.485235 | -24.1124 | -2.65684×10-3 | 1.29223×10-7 | 0 | 0 | 0 | 52478 |
| 1358~3200 | -13542.026 | 183.803828 | -31.38 | 0 | 0 | 0 | 0 | 3.642×1029 | 0 | |
Tetr In |
298.15~429.75 | -6978.89 | 92.338115 | -21.8386 | -5.72566×10-3 | -2.120321×10-6 | 0 | 0 | 0 | -22906 |
| 429.75~3800 | -7033.516 | 124.476588 | -27.4562 | -0.54607×10-3 | -0.08367×10-6 | 0 | 0 | 3.53×1022 | -211708 |
* a~i: G
2.2 液相, 富铜固溶体相
液相 (Liquid) 、 富铜固溶体相 (α) 均采用替代模型描述, 摩尔自由焓表达式如下:
其中xi为组分i的摩尔分数, °G
2.3 线性化合物: Cu11In9
Cu11In9相采用双亚晶格 (Cu) 0.55 (In) 0.45模型描述, 摩尔自由焓的数学表达式为:
GCu11In9=0.55 °G
其中a, b为待优化系数。
2.4 非线性化合物: β, δ, γ, η, η′
β, δ, γ, η, η′相都是有一定成分范围的非线性化合物, 其成分和温度范围列于表2。 根据γ, η′相结构的特点
G=y″Cu°GCu∶Cu∶In+y″In°GCu∶In∶In+y″Va°GCu∶Va∶In+x″RT{y″Culn (y″Cu) +y″Inln (y″In) +y″Valn (y″Va) }+y″Cuy″InLCu∶Cu, In∶In+y″Cuy″VaLCu∶Cu, Va∶In+y″Iny″VaLCu∶In, Va∶In (J·mol-1) (4)
其中x″表示第二个亚晶格所占晶格阵点的摩尔分数, y″i表示组分i在第二个亚晶格中的摩尔分数, LCu∶i, j∶In表示第二个亚晶格中i, j组元间的相互作用参数。
3 参数优化及结果
本文采用Thermo-Calc的Parrot优化模块
表2 非线性化合物的成分和温度范围
Table 2 Composition and temperature range of non-stoichiometric compounds
| Phase | Composition range/ (% In) | Temperature range/K | Refs. |
β |
18.05~24.5 18.2~23.8 |
847~983 847~988 |
[3] [2] |
δ |
28.9~30.6 | 298~904 | [1] |
γ |
27.7~31.3 | 887~957 | [1] |
η′ |
32.92~37.8 | 550~942 | [1] |
η |
35.2~37.8 | 298~662 | [8] |
表3 Cu-In二元系中各相热力学参数的优化结果
Table 3 Optimized parameters for Cu-In system (J·mol-1)
| Phase | Model | Parameters in this work |
Liquid |
(Cu, In) | L(0)Cu,Ιn=-41545.0+238.89371T-29.90241TlnT |
| L(1)Cu,Ιn=-79490.5+376.06364T-44.9521TlnT | ||
| L(2)Cu,Ιn=-41279.9+186.07952T-22.65452TlnT | ||
α |
(Cu, In) | L(0)Cu,Ιn=2191.2+14.44526T |
| L(1)Cu,Ιn=-42307.2+0.77762T | ||
| L(2)Cu,Ιn=-55686.5-39.73087T | ||
β |
(Cu) 0.73 (Cu, In, Va) 0.14 (In) 0.13 | °GCu∶Cu∶In-0.87°GfccCu-0.13°GtetrΙn=-115.5501-3.19348T |
| °GCu∶I∶In-0.73°GfccCu-0.27°GtetrIn=-2331.64-3.89391T | ||
| °GCu∶Va∶In-0.73°GfccCu-0.13°GtetrIn=5326.035-4.63147T | ||
γ |
(Cu) 0.654 (Cu, In, Va) 0.115 (In) 0.231 | °GCu∶Cu∶In-0.769°GfccCu-0.231°GtetrΙn=-4950.02-0.343T |
| °GCu∶In∶In-0.654°GfccCu-0.346°GtetrΙn=-6217.547-0.88817T | ||
| °GCu∶Va∶In-0.654°GfccCu-0.231°GtetrΙn=5000 | ||
δ |
(Cu) 0.68 (Cu, In, Va) 0.04 (In) 0.28 | °GCu∶Cu∶In-0.72°GfccCu-0.28°GtetrΙn=-7703.608+1.3553T |
| °GCu∶In∶In-0.68°GfccCu-0.32°GtetrIn=-7991.308+1.1853T | ||
| °GCu∶Va∶In-0.68°GfccCu-0.28°GtetrΙn=5000 | ||
| L(0)Cu∶Cu∶Ιn∶Ιn=-467.2+0.5T | ||
η′ |
(Cu) 0.545 (Cu, In, Va) 0.122 (In) 0.333 | °GCu∶Cu∶In-0.667°GfccCu-0.333°GtetrΙn=-6372.397-0.8396T |
| °GCu∶Cr∶In-0.545°GfccCu-0.455°GtetrΙn=-168.969-7.0597T | ||
| °GCu∶Cu∶In-0.545°GfccCu-0.333°GtetrΙn=5000 | ||
| L(0)Cu∶Cu∶Ιn∶Ιn=-14526.546+18.02T | ||
η |
(Cu) 0.62 (Cu, In, Va) 0.03 (In) 0.35 | °GCu∶Cu∶In-0.65°GfccCu-0.35°GtetrΙn=-8125.223+1.355T |
| °GCu∶In∶In-0.62°GfccCu-0.38°GtetrIn=-7923.823+1.38T | ||
| °GCu∶In∶In-0.62°GfccCu-0.35°GtetrΙn=5000 | ||
Cu11In9 |
(Cu) 0.55 (In) 0.45 | °GCu11In9-0.55°GfccCu-0.45°GtetrΙn=-7105.055+0.9533T |
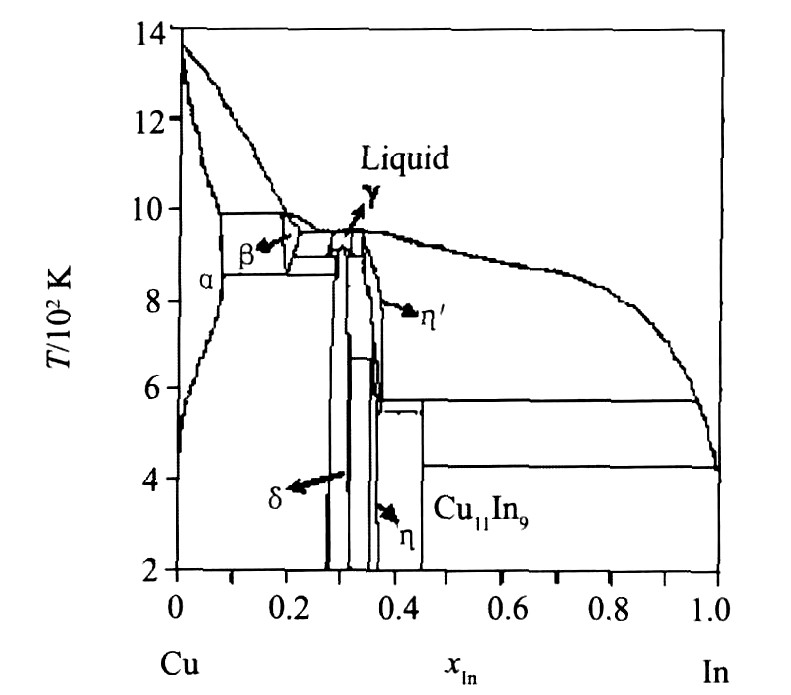
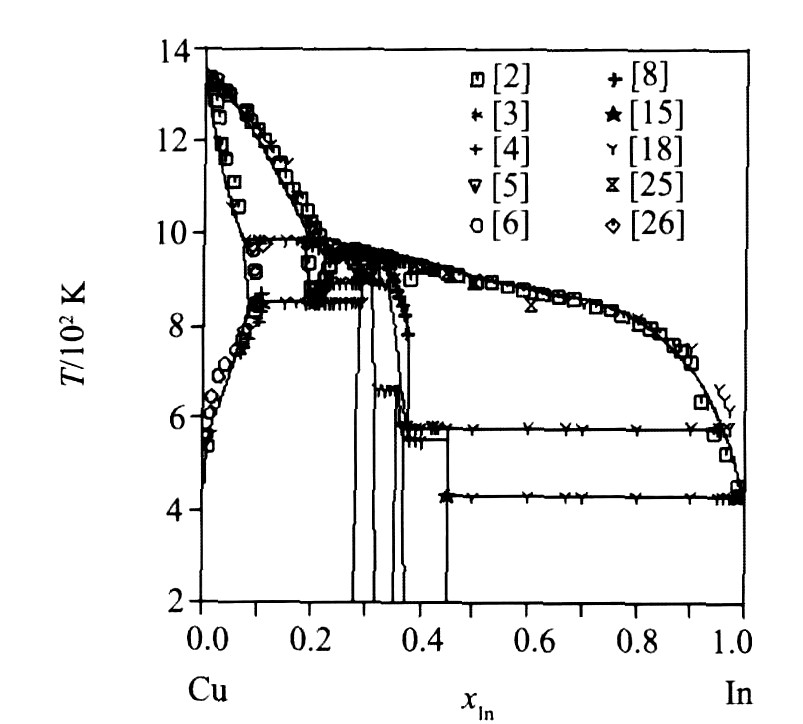
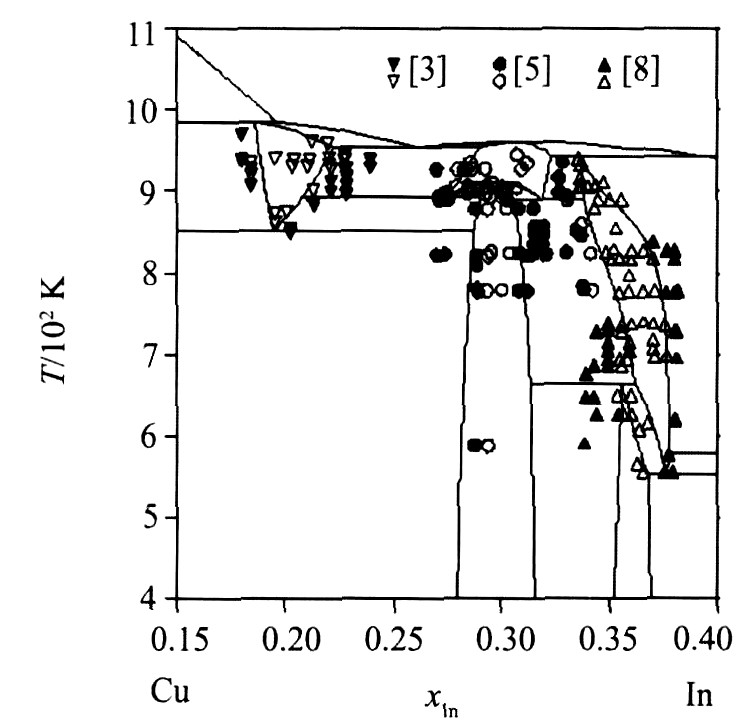
图1为由各相热力学参数优化结果计算的相图, 图2为计算相图与文献实验相平衡数据的比较, 图3为非线性化合物相区和实验结果的比较, 表4对比了无变反应计算值和文献实验值。 通过以上比较, 看出计算相图与已有的相平衡数据吻合很好, 非线性化合物的组分范围也与文献实验数据基本一致。
图1 Cu-In二元系的计算相图
Fig.1 Calculated phase diagram of Cu-In binary system
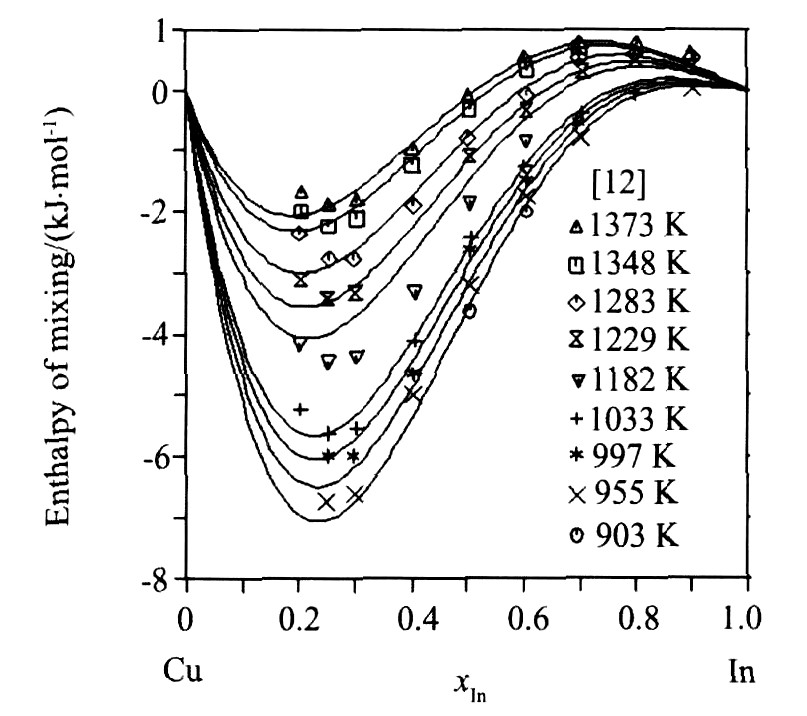
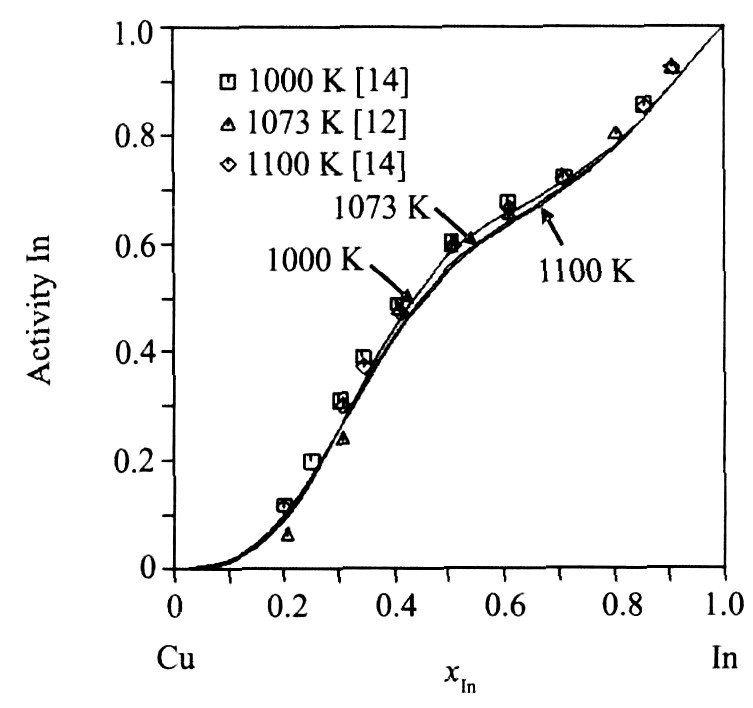
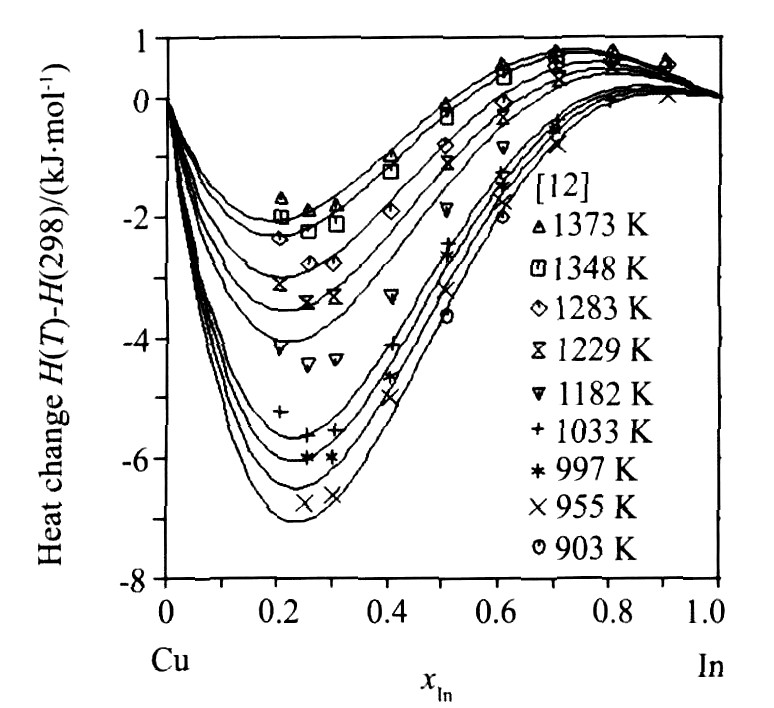
图4为利用优化结果计算的液相豁合焓与实验数据的比较, 图5为液相中In活度的计算值与实验值的符合情况, 图6为Cu0.7In0.3合金的摩尔焓计算值与实验值的比较。 从图4~6可以看出, 由优化结果计算的热力学性质与实验值均能很好地吻合。
图2 计算相图与实验相平衡数据的比较
Fig.2 Comparison of calculated phase diagram with experimental data
表4 计算相图中的无变反应与实验数据的比较
Table 4 Calculated invariant reactions compared with experimental data
| Reaction | Composition (% In) | Temperature/K | Refs. | ||
| Liquid+α?β |
20.8 ~20.9 20.0 19.6 |
9.5 10.05 7.5 7.18 |
18.3 18.05 19.0 18.6 |
988 983 984.5 984.0 |
[2] [3] [18] This work |
Liquid?β+γ |
25.8 - 25.5 26.0 26.0 |
23.8 24.5 - 22.0 22.2 |
27.2 - 27.7 28.5 28.5 |
952 949 950 952.2 952.9 |
[2] [3] [5] [18] This work |
Liquid?γ |
29.1 29.56 29.4 30.3 |
29.1 29.56 29.4 30.3 |
- - - - |
958.2 955.5 957.3 960.2 |
[2] [5] [18] This work |
Liquid+γ?η′ |
35.2 - 35.4 35.6 36.6 |
31.2 31.3 - 32.0 31.6 |
32.7 32.9 33.0 33.3 33.6 |
944 940 940 943.4 942.4 |
[2] [5] [8] [18] This work |
γ?β+δ |
27.9 27.7 - 27.0 |
22.1 21.8 22.0 20.9 |
28.2 28.9 29.0 29.1 |
889 890 893.3 889.6 |
[2] [5] [18] This work |
γ?δ |
29.5 30.15 29.8 29.8 |
29.5 30.15 29.8 29.8 |
- - - - |
904.2 903.2 905.4 907.2 |
[2] [5] [18] This work |
γ?δ+η′ |
30.8 31.3 - 32.0 31.9 |
30.5 30.6 - 30.6 30.6 |
32.7 33.1 33.4 33.3 33.8 |
888 886 887 891.0 887.8 |
[2] [5] [8] [18] This work |
β?α+δ |
20.0 20.15 - - 19.0 19.5 |
11.63 10.90 10.90 10.85 - 8.29 |
28.7 - - - 29.0 28.8 |
847 847 848 848 849.7 848.7 |
[2] [3] [4] [6] [18] This work |
η′+δ?η |
35.0 - 36.2 |
32.0 - 31.4 |
34.5 - 35.6 |
661.8 662 661.9 |
[18] [25] This work |
η′+Liquid?Cu11In9 |
36.4 37.8 37.7 |
97.0 97.0 95.8 |
45.0 45.0 45.0 |
579.0 580 580.4 |
[18] [25] This work |
η′?η+Cu11In9 |
- 37.6 |
- 36.8 |
- 45.0 |
549.8 549.7 |
[18] This work |
Liquid?Cu11In9+In |
- 98.4 99.3 |
- 45.0 45.0 |
100 100 100 |
428.7 427 430.2 |
[18] [25] This work |
图3 局部相图中相区内的实验数据的吻合情况 (实心点为落在两相区内的点, 空心点为落在单相区内的点)
Fig.3 Comparison of calculated phase diagram from 15% to 40% In with selected data (solid symbols in two-phase regions, open symbols in single-phase regions)
图4 Cu-In体系的液相混合焓的计算值与实验数据的比较
Fig.4 Comparison between calculated enthalpies of mixing of Cu-In liquid phase and experimental data
图5 Cu-In体系液相中In活度计算值与实验数据的比较 (参考态: 液相纯In)
Fig.5 Comparison between calculated activities of indium in Cu-In liquid phase and experimental data (reference state: liquid In)
图6 Cu0.7In0.3合金摩尔焓的计算结果与实验数据的比较
Fig.6 Comparison between calculated molar enthalpies of the Cu0.7In0.3 alloy and experimental data
4 结 论
对Cu-In二元系进行了热力学优化, 选用了适合的热力学模型描述体系中的各相, 利用相关实验数据, 优化出各相的热力学模型参数。 用优化结果计算的相图以及热力学性质与实验数据吻合较好。
参考文献
[10] Rajasekharan T P, Schubert K.Crystal structure of Cu11In9[J].Z.Metallkd., 1981, 72:275.
[19] Dinsdale A T.SGTE data for pure elements[J].Calphad, 1991, 15:317.
[20] Sundman B, Jansson B, Anderson J O.Thermocalc databank sys-tem[J].Calphad, 1985, 9:153.
[21] 储茂友, 郭江贵, 商顺利, 孙军, 沈剑韵.W-Zr体系的热力学优化[J].稀有金属, 2003, 27 (6) :701.
[26] Okamoto H.Cu-In (copper-indium) [J].J.Phase Equilibria, 1991, 12 (6) :702.
[10] Rajasekharan T P, Schubert K.Crystal structure of Cu11In9[J].Z.Metallkd., 1981, 72:275.
[19] Dinsdale A T.SGTE data for pure elements[J].Calphad, 1991, 15:317.
[20] Sundman B, Jansson B, Anderson J O.Thermocalc databank sys-tem[J].Calphad, 1985, 9:153.
[21] 储茂友, 郭江贵, 商顺利, 孙军, 沈剑韵.W-Zr体系的热力学优化[J].稀有金属, 2003, 27 (6) :701.
[26] Okamoto H.Cu-In (copper-indium) [J].J.Phase Equilibria, 1991, 12 (6) :702.