Trans. Nonferrous Met. Soc. China 23(2013) 2153-2159
Effects of Acidiphilium cryptum on biosolubilization of rock phosphate in the presence of Acidithiobacillus ferrooxidans
Chun-qiao XIAO, Ru-an CHI, Yu-juan FANG
Key Laboratory for Green Chemical Process of Ministry of Education, Wuhan Institute of Technology, Wuhan 430073, China
Received 27 June 2012; accepted 15 October 2012
Abstract:
The bioleaching of pyrite and biosolubilization of rock phosphate (RP) in 9K basal salts medium were compared by the following strains of an autotrophic acidophilic bacterium, Acidithiobacillus ferrooxidans, a heterotrophic acidophilic bacterium, Acidiphilium cryptum, and mixed culture of At. ferrooxidans and A. cryptum. The results show that A. cryptum is effective in enhancing the bioleaching of pyrite and biosolubilization of RP in the presence of At. ferrooxidans, although it could not oxidize pyrite and solubilize RP by itself. This effect is demonstrated experimentally that A. cryptum enhances a decrease in pH and an increase in redox potential, concentration of total soluble iron and planktonic part bacterial number in the broth during pyrite bioleaching processes by At. ferrooxidans. The mixed culture of At. ferrooxidans and A. cryptum leads to the most extensive soluble phosphate released at 30 °C. Pulp density exceeding 3% is shown to adversely influence the release of soluble phosphate by the consortium of At. ferrooxidans and A. cryptum. It is essential to add pyrite to the 9K basal salts medium for the biosolubilization of RP by the mixed culture of At. ferrooxidans and A. cryptum, and the percentage of soluble phosphate released is the greatest when the mass ratio of RP to pyrite is 1:2 or 1:3.
Key words:
Acidiphilium cryptum; bioleaching; pyrite; rock phosphate; biosolubilization; Acidithiobacillus ferrooxidans;
1 Introduction
Phosphorus is one of the most important essential elements for crop production. Despite phosphorus being widely and abundantly distributed in the soils in both its inorganic and organic forms, many soils throughout the world are deficient in phosphorus. Hence, large amounts of soluble phosphate are applied to soils as fertilizer for sustaining profitable agricultural production.
Natural rock phosphate (RP) is a complex raw material and is mainly used in the manufacture of phosphate fertilizer [1]. Almost 80% of RP all over the world is low-grade and not suitable for direct application to soils as a phosphate fertilizer because of its low phosphorus content and poor solubility [2]. Conventionally, RP is chemically processed with sulfuric acid or phosphoric acid into phosphate fertilizer. This process makes the fertilizer more expensive and contributes to environmental pollution [3]. Consequently, a more efficient process to release soluble phosphate from RP is being sought.
As an alternative to the direct use of sulfuric or phosphoric acid, microorganisms may be considered a source of solubilizing agents for insoluble mineral phosphates [4]. Some microorganisms, including bacteria and fungi, are known to be involved in the solubilization of RP [5-7]. These phosphate-solubilizing micro- organisms used for industrial production of phosphate fertilizer lower the production cost. Their activity may also be exploited when insoluble mineral phosphate is applied directly to soils [8,9].
Autotrophic acidophilic bacteria have special interest recently as phosphate-solubilizing micro-organisms because these bacteria, including iron- and sulfur-oxidizing microorganisms, are commonly used in bioleaching and play an important role in hydrometallurgy because of the ease of handling them, the low cost of their application, and the ability to control the impact they have on the environment [10]. Owing to the numerous applications of autotrophic acidophilic bacteria in the bioleaching of sulfide minerals, several studies have been done with autotrophic acidophilic bacteria, such as Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans, to solubilize RP [11-13]. However, most of the earlier experiments with autotrophic acidophilic bacteria were performed on a small scale. Although RP can be solubilized successfully by these bacteria, the low release of soluble phosphate was a major challenge. Thus, enhancement of the release of soluble phosphate from RP would make the use of RP in agriculture more effective and economic.
At. ferrooxidans is most studied in the bioleaching acidophiles and was thought to be the most important contributor to enhance bioleaching, largely because it flourished in laboratory cultures. However, its ability to oxidize ferrous ion and/or sulfur is repressed to different degrees by the addition of naturally-occurring organic compounds in growth media [14]. Several heterotrophic microorganisms have been reported to remove this inhibition by metabolizing the organic materials, consequently enhance the growth of the autotrophs and as a result improve the leaching rate of metal sulfides [15]. In this study, a heterotrophic acidophilic bacterium Acidiphilium cryptum was introduced into an autotrophic acidophilic bacterium At. ferrooxidans for the bioleaching of pyrite and biosolubilization of RP in the presence of pyrite. The effects of temperature, pulp density and mass ratio of RP to pyrite on the biosolubilization of RP by the consortium of At. ferrooxidans and A. cryptum were also investigated.
2 Experimental
2.1 Bacteria and culturing techniques
The bacteria used in this experiment were autotrophic acidophilic bacterium At. ferrooxidans and heterotrophic acidophilic bacterium A. cryptum, which were kindly supplied by Central South University (Changsha, China). At. ferrooxidans was routinely cultured in 250 mL flasks containing 100 mL 9K basal salts medium with 44.2 g/L FeSO4·7H2O as an energy source [16], and previously adjusted to pH of 2.5 using 20% sulfuric acid. A. cryptum was grown in 250 mL flasks containing 100 mL 9K basal salts medium with 1 g/L glucose and 0.3 g/L yeast extract as an energy source, and adjusted to initial pH of 3.5 using 20% sulfuric acid. Flasks were incubated at 30 °C on a rotary shaker at 160 r/min. The cultures that were used had been subcultured through five transfers in pyrite- containing medium in order to adapt the bacteria under the experimental conditions.
2.2 Assays of bioleaching of pyrite
The pyrite sample was obtained from Daye iron mines (Hubei, China), which mainly contained 90% FeS2 and some quartz. The pyrite was crushed, ground and dry-sieved to a particle size of 75-147 μm. Prior to use, the pyrite was washed with 2 mol/L hydrochloric acid to remove any surface oxidized deposits, rinsed repeatedly with distilled water, and then dried at 50 °C for 24 h and sterilized by UV for 24 h. Bioleaching of pyrite was carried out in 250 mL flasks with 100 mL 9K basal salts medium containing 2 g pyrite. The pH of the medium was initially adjusted to 2.5 except for that of the pure culture of A. cryptum, which was adjusted to pH 3.5. The cell density of each culture was adjusted previously at 1×107 cell/mL and inoculated at 10% (v/v). Cultures of At. ferrooxidans and A. cryptum were mixed at 1:1 (v/v) for the assays of the consortium. All the assays were performed at 30 °C on a rotary shaker at 160 r/min for 14 d, and samples were taken out every day to determine pH, redox potential, total soluble iron concentration and planktonic part bacterial number. Autoclaved, uninoculated medium containing 20 g/L pyrite was served as abiotic control. All experiments were performed in triplicate.
2.3 Assays of biosolubilization of RP
The RP sample was obtained from Yichang phosphate mines (Hubei, China), and was crushed, ground and dry-sieved to a particle size of 75-147 μm. X-ray diffraction analysis showed that the sample was mainly composed of hydroxyapatite and a small quantity of quartz and montmorillonite. Biosolubilization of RP was tested in 250 mL flasks with 100 mL 9K basal salts medium containing 2 g pretreated pyrite and 1 g RP (mass ratio of RP to pyrite: 1:2, pulp density: 3% (w/v), the same below if not mentioned). The pH of the medium was initially adjusted to 2.5 except for that of the pure culture of A. cryptum, which was adjusted to 3.5. The cell density of each culture was adjusted previously at 1×107 cell/mL and inoculated at 10% (v/v). Cultures of At. ferrooxidans and A. cryptum were mixed at 1:1 (v/v) for the assays of the consortium. All the assays were performed at 30 °C on a rotary shaker at 160 r/min for 14 d, and samples were taken every day and filtered through a 0.45 μm pore-size filter paper. Then the filtrate was centrifuged at 11000 ×g for 20 min, and the supernatant was assayed for the content of soluble phosphate. Autoclaved, uninoculated medium containing 20 g/L pyrite and 10 g/L RP was served as abiotic control. To investigate the optimal temperature for the solubilization of RP by the consortium of At. ferrooxidans and A. cryptum, the flasks were shaken at different temperatures (20, 25, 30, 35, and 40 °C, respectively). Subsequently, the effect of pulp density (w/v) was studied at 1%, 2%, 3%, 4%, and 5% (w/v), respectively. Finally, the effect of mass ratio of RP to pyrite (3:1, 2:1, 1:1, 1:2, and 1:3, respectively) in the broth on RP biosolubilization was studied. All experiments were performed in triplicate.
2.4 Analytical methods
The pH value was recorded with a pH meter equipped with glass electrode. The redox potential was measured using a platinum electrode combined with Ag/AgCl reference electrode and converted to Eh values (i.e. relative to a hydrogen reference electrode) by adding 234 mV to the measured values [17]. Total soluble iron was determined by atomic absorption spectrometry. The planktonic part of the bacterial number was counted by means of a haemocytometer under microscope. Content of soluble phosphate was determined by the vanadium- ammonium molybdate colorimetric method with a UV-vis 8500 spectrophotometer at 490 nm [18]. Values were given as mean ± standard deviation for triplicate samples.
3 Results and discussion
3.1 Bioleaching of pyrite by At. ferrooxidans and A. cryptum
The bioleaching of pyrite has been studied extensively, and recent work has provided strong evidence that the pyrite bioleaching by autotrophic acidophilic bacteria occurs via a multiple subprocess mechanism [19-22].
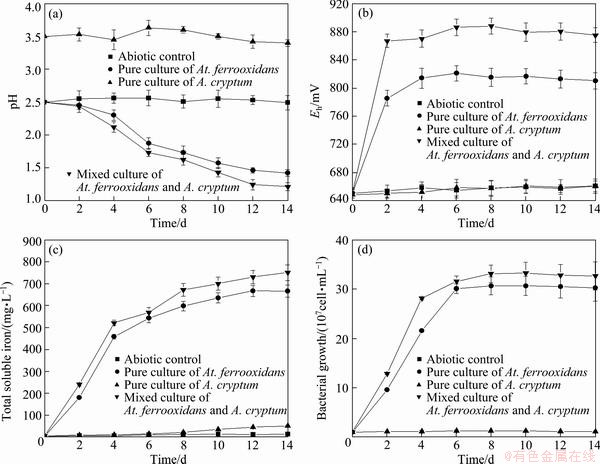
Fig. 1 Changes in pH (a), redox potential (b), concentration of total soluble iron (c), and planktonic part bacterial number (d) in broth during pyrite bioleaching processes by At. ferrooxidans and A. cryptum
In this study, the dynamics of the bioleaching of pyrite was followed by determining the pH, redox potential, concentration of total soluble iron and planktonic part bacterial number in the broth. The results obtained in 14 d experiments are shown in Fig. 1. The pH in the broth decreased gradually after being inoculated with At. ferrooxidans, and at the end of the experiment, the pH decreased to 1.44 from an initial pH of 2.5 (Fig. 1(a)). An obvious increase of redox potential in the biotic test was consistent with the oxidizing activity of At. ferrooxidans. This trend was confirmed by the measured Eh, which increased from 651 to 825.7 mV in the At. ferrooxidans culture (Fig. 1(b)). The activity was also manifested through the increase of the concentration of total soluble iron in the broth, in which the highest concentration of 680.3 mg/L was obtained (Fig. 1(c)). Furthermore, At. ferrooxidans grew well in the presence of pyrite, and the bacterial abundance increased after inoculation and reached a maximum of about 3.1 ×108 cell/mL after 14 d (Fig. 1(d)). However, the changes of pH, redox potential and concentration of total soluble iron in the abiotic control were negligible.
Autotrophic acidophilic bacteria can obtain energy for growth by oxidizing Fe (II) and/or reduced forms of sulfur [23,24]. However, their oxidizing activity is always repressed to different degrees by the production of organic compounds in the broth [25]. It is interesting to note that some heterotrophic acidophilic bacteria, which are able to metabolize organic compounds as a source of energy and detoxify the growth environment for autotrophic acidophilic bacteria, were reported to form a mutualism with autotrophic acidophilic bacteria in mineral bioleaching process [26-29]. In this study, a heterotrophic acidophilic bacterium, A. cryptum, was added to the broth as it was hypothesized that it could cooperate with At. ferrooxidans by feeding on organic excretions from the latter. This heterotroph does not oxidize iron(II) or sulfur as sole energy substrate but able to reduce Fe3+ and metabolize a small amount of organic compounds, such as glucose and yeast extract, but a large amount of organic compounds inhibit its growth [14].
Figure 1(a) shows that the mixed culture of At. ferrooxidans and A. cryptum appeared to reduce the pH more effectively than the pure culture of At. ferrooxidans, which is decreased by 8.3% (compared with the pH at the end of the experiment). Results also show that A. cryptum was able to improve the bioleaching of pyrite by At. ferrooxidans, and the redox potential and concentration of total soluble iron were elevated by 7.1% and 10.7%, respectively, at the end of the experiment in the presence of A. cryptum (Figs. 1(b) and (c)). The planktonic part bacterial number in the broth inoculated with mixed culture of At. ferrooxidans and A. cryptum was higher than that with pure culture of At. ferrooxidans (Fig. 1(d)). The result indicated that although A. cryptum did not grow in the medium without any organic matter addition, it was able to enhance the cell growth of At. ferrooxidans. It also displayed a fast growth of the mixture of At. ferrooxidans and A. cryptum, which was 10.7% higher than the rate observed for the pure culture of At. ferrooxidans. However, as for the pure culture of A. cryptum, since it was not able to oxidize pyrite as its growth source, it did not grow well in the broth. These results suggest that the presence of a consortium of At. ferrooxidans and A. cryptum may determine a higher pyrite oxidizing efficiency due to a coupled and synergistic metabolism [30]. Heterotrophs were reported to assist bioleaching environments by metabolizing organic matter generated by autotrophs, and thus detoxifying the growth environment for autotrophs [31]. At the same time the growth of autotrophs and the release of labile organic material from their metabolism seem to be enough for the heterotrophic needs of the Fe-reducing bacteria.
3.2 Biosolubilization of RP by At. ferrooxidans and A. cryptum
Autotrophic acidophilic bacteria have a key role in the production of sulfuric acid from the oxidation of pyrite. The sulfuric acid creates an acid environment and thus benefits the solubilization of RP. The hydroxyapatite, which is the major mineral present in the RP sample, is solubilized according to the following chemical reaction:
5H2SO4+Ca5(OH)(PO4)3→5CaSO4+3H3PO4+H2O
In this study, both the pure culture of At. ferrooxidans and the mixed culture of At. ferrooxidans and A. cryptum could effectively release soluble phosphate from RP compared with the abiotic control, and the addition of pyrite was very important to the biosolubilization of RP (Fig. 2(a)). At the end of the experiment, the pyrite-added system achieved the highest percentage of soluble phosphate released of 26.1% and 28.5%, respectively, for the pure culture of At. ferrooxidans and mixed culture of At. ferrooxidans and A. cryptum, compared with 4.3% and 4.4%, respectively, for the same system without the additional pyrite. The mixed culture of At. ferrooxidans and A. cryptum released more soluble phosphate (9.2% elevated at the end of the experiment) compared with the pure culture of At. ferrooxidans. This indicated that the application of the heterotrophic acidophilic bacterium, A. cryptum, was an effective method to promote the biosolubilization of RP by the autotrophic acidophilic bacterium At. ferrooxidans. It seems to be a feasible and attractive approach to enhance the soluble phosphate releasing efficiency from RP with coinoculation of At. ferrooxidans and A. cryptum.
Figure 2(b) shows that although there is obvious increase of pH as a result of the consumption of acid by the proton attack on RP for the pure culture of At. ferrooxidans and mixed culture of At. ferrooxidans and A. cryptum, respectively, at the 4th day, gradual decrease of pH was observed after inoculation for 4 d. However, no pH reduction but sharp increase of pH was observed in abiotic control or in the biotic test without pyrite addition during the experiment. It indicated that the pyrite addition had a significant influence on the bacterial performance. Results also exhibited that the pH of the broth inoculated with the mixed culture of At. ferrooxidans and A. cryptum was lower than that inoculated with the pure culture of At. ferrooxidans during the solubilizing processes, and decreased by 9.6% in pH at the end of the experiment. The heterotrophic acidophilic bacterium A. cryptum could improve the pH-reducing ability of autotrophic acidophilic bacterium At. ferrooxidans, since it was not able to reduce pH by itself.
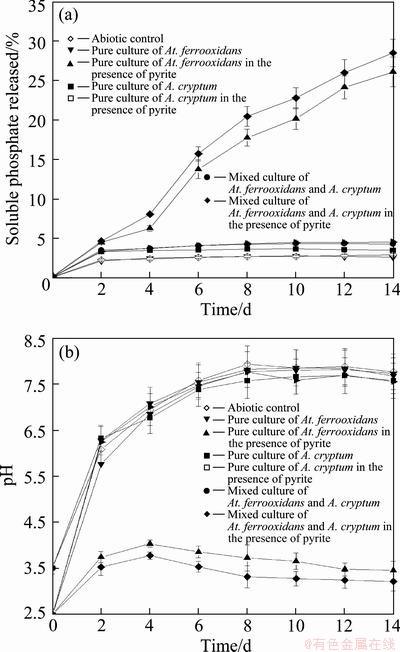
Fig. 2 Changes in percentage of soluble phosphate released (a) and pH (b) during RP solubilizing processes by At. ferrooxidans and A. cryptum
3.3 Optimization of temperature, pulp density, and mass ratio of RP to pyrite for biosolubilization of RP by consortium of At. ferrooxidans and A. cryptum
Biosolubilization of RP is a complex natural process affected by a number of factors including temperature, pulp density, pH, particle size, presence of toxic elements etc., controlling the activity of bacteria and the chemistry of the solubilizing process. Therefore, the effects of temperature, pulp density, and mass ratio of RP to pyrite on the biosolubilization of RP by the mixed culture of At. ferrooxidans and A. cryptum were investigated.
The effect of temperature on the soluble phosphate released during 14 d of RP solubilizing experiments with mixed culture of At. ferrooxidans and A. cryptum in the presence of pyrite is shown in Fig. 3. Results show that the optimal temperature for the biosolubilization of RP by the consortium of At. ferrooxidans and A. cryptum was 30 °C, and when the temperature was higher or lower than the optimal temperatures, the percentage of soluble phosphate released decreased.
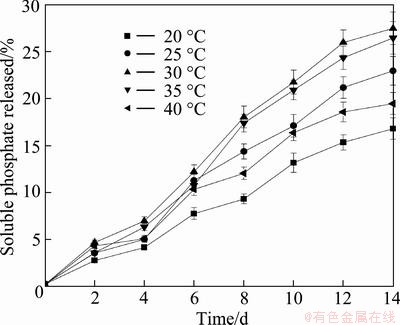
Fig. 3 Effect of temperature on soluble phosphate released during RP solubilizing processes by mixed culture of At. ferrooxidans and A. cryptum in the presence of pyrite
Figure 4 shows the greatest percentage of soluble phosphate released during 14 d of RP solubilizing experiments by mixed culture of At. ferrooxidans and A. cryptum in the presence of pyrite at a pulp density of 3% (w/v). Increasing pulp density exceeding by 3% adversely influenced the biosolubilization of RP. The adverse effect of increasing pulp density could be attributed to the inhibitory effect of increasing concentrations of ferric iron, the limited availability of nutrients and, O2 and CO2 with increasing pulp density and the mechanical damage to bacterial cells by solids [32-34].
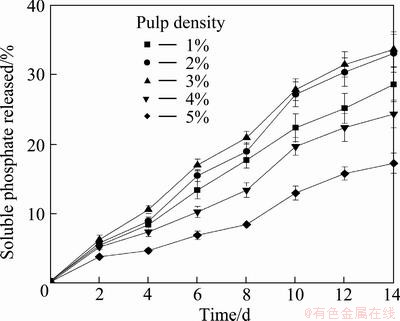
Fig. 4 Effect of pulp density on soluble phosphate released during RP solubilizing processes by mixed culture of At. ferrooxidans and A. cryptum in the presence of pyrite
High mass ratio of RP to pyrite had a negative influence on biosolubilization of RP as illustrated in Fig. 5. Percentage of soluble phosphate released was the greatest and nearly similar in extent when the mass ratio of RP to pyrite was 1:2 or 1:3. Pyrite was the energy source for the growth of At. ferrooxidans during the RP solubilizing process, therefore, relatively low mass ratio of RP to pyrite was more effective in the biosolubilization of RP by the consortium of At. ferrooxidans and A. cryptum.
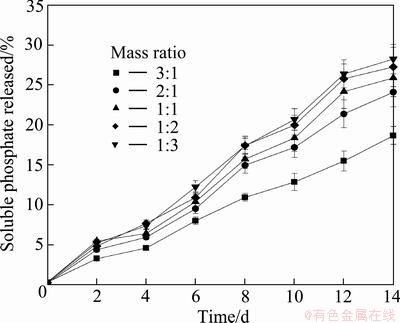
Fig. 5 Effect of mass ratio of RP to pyrite on soluble phosphate released during RP solubilizing processes by mixed culture of At. ferrooxidans and A. cryptum in the presence of pyrite
4 Conclusions
1) A heterotrophic acidophilic bacterium A. cryptum was found to enhance the bioleaching of pyrite by the autotrophic acidophilic bacterium At. ferrooxidans. A combined bioleaching experiment of pyrite (coupling At. ferrooxidans and A. cryptum) indicated that the lowering of pH and the total soluble iron released from pyrite were significantly improved. As a result the redox potential was elevated, which benefited bacterial growth.
2) The introduction of A. cryptum into the RP solubilizing system by At. ferrooxidans was manifested experimentally to improve the RP solubilizing efficiency of At. ferrooxidans.
3) Effects of temperature, pulp density and mass ratio of RP to pyrite on biosolubilization of RP in pyrite-containing 9K basal salts medium inoculated with the mixed culture of At. ferrooxidans and A. cryptum were investigated, and the maximum percentage of soluble phosphate released was recorded at temperature 30 °C, pulp density 3% and mass ratio of RP to pyrite 1:2 or 1:3, respectively.
References
[1]  H, FRAGA R. Phosphate solubilizing bacteria and their role in plant growth promotion [J]. Biotechnol Adv, 1999, 17: 319-359.
H, FRAGA R. Phosphate solubilizing bacteria and their role in plant growth promotion [J]. Biotechnol Adv, 1999, 17: 319-359.
[2] RAJAN S S S, WATKINSON J H, SINCLAIR A G. Phosphate rocks for direct application to soils [J]. Adv Agron, 1996, 57: 77-159.
[3] VASSILEV N, MEDINA A, AZCON R, VASSILEVA M. Microbial solubilization of rock phosphate on media containing agro-industrial wastes and effect of the resulting products on plant growth and P uptake [J]. Plant Soil, 2006, 287: 77-84.
[4] SPERBER J I. Solution of mineral phosphates by soil bacteria [J]. Nature, 1957, 180: 994-995.
[5] ANTOUN H, BABANA A H. Effect of Tilemsi phosphate rock-solubilizing microorganisms on phosphorus uptake and yield of field-grown wheat (Triticum aestivum L.) in Mali [J]. Plant Soil, 2006, 287: 51-58.
[6] HAMDALI H, HAFIDI M, VIROLLE M J, OUHDOUCH Y. Rock phosphate solubilizing actinomycetes: Screening for plant growth promoting activities [J]. World J Microbiol Biotechnol, 2008, 24: 2565-2575.
[7] XIAO C Q, CHI R A, HE H, QIU G Z, WANG D Z, ZHANG X W. Isolation of phosphate-solubilizing fungi from phosphate mines and their effect on wheat seedling growth [J]. Appl Biochem Biotechnol, 2009, 159: 330-342.
[8] ZHU H J, SUN L F, ZHANG Y F, ZHANG X L, QIAO J J. Conversion of spent mushroom substrate to biofertilizer using a stress-tolerant phosphate-solubilizing Pichia farinose FL7 [J]. Bioresour Technol, 2012, 111: 410-416.
[9] CHEN Y P, REKHA P D, ARUN A B, SHEN F T, LAI W A, YOUNG C C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities [J]. Appl Soil Ecol, 2006, 34: 33-41.
[10] EHRLICH H L. Past, present and future of biometallurgy [J]. Hydrometallurgy, 2001, 59: 127-134.
[11] CHI R A, XIAO C Q, GAO H. Bioleaching of phosphorus from rock phosphate containing pyrites by Acidithiobacillus ferrooxidans [J]. Miner Eng, 2006, 19: 979-981.
[12] STAMFORD N P, SANTOS P R, SANTOS C E S, FREITAS A D S, DIAS S H L, LIRA M A Jr. Agronomic effectiveness of biofertilizers with phosphate rock, sulphur and Acidithiobacillus for yam bean grown on a Brazilian tableland acidic soil [J]. Bioresour Technol, 2007, 98: 1311-1318.
[13] BHATTI T M, YAWAR W. Bacterial solubilization of phosphorus from phosphate rock containing sulfur-mud [J]. Hydrometallurgy, 2010, 103: 54-59.
[14] WATLING H R, PERROT F A, SHIERS D W. GROSHEVA A, RICHARDS T N. Impact of the copper solvent extraction reagent LIX 984N on the growth and activity of selected acidophiles [J]. Hydrometallurgy, 2009, 95: 302-307.
[15] XU Ai-ling, XIA Jin-lan, ZHANG Shuai, YANG Yu, NIE Zhen-yuan, QIU Guan-zhou. Bioleaching of chalcopyrite by UV-induced mutagenized Acidiphilium cryptum and Acidithiobacillus ferrooxidans [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(2): 315-321.
[16] SILVERMAN M P, LUNDGREN D G. Studies on the chemoautotrophic iron bacterium Thiobacillus ferrooxidans: I. An improved medium and a harvesting procedure for securing high cellular yields [J]. J Bacteriol, 1959, 77: 642-647.
[17] YAHYA A, JOHNSON D B. Bioleaching of pyrite at low pH and low redox potentials by novel mesophilic Gram-positive bacteria [J]. Hydrometallurgy, 2002, 63: 181-188.
[18] JIANG Li-hong, ZHAO Sheng-lan, ZHU Jia-hua. Fast destination of P2O5 in melamine phosphate [J]. Chem Eng, 2001, 85(4): 50-51. (in Chinese)
[19] ZHANG Lin, QIU Guan-zhou, HU Yue-hua, SUN Xiao-jun, LI Jian-hua, GU Guo-hua. Bioleaching of pyrite by A. ferrooxidans and L. ferriphilum [J]. Transactions of Nonferrous Metals Society of China, 2008, 18: 1415-1420.
[20] XIA Le-xian, TANG Lu, XIA Jin-lan, YIN Chu, CAI Li-yuan, ZHAO Xiao-juan, NIE Zhen-yuan, LIU Jian-she, QIU Guan-zhou. Relationships among bioleaching performance, additional elemental sulfur, microbial population dynamics and its energy metabolism in bioleaching of chalcopyrite [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(1): 192-198.
[21] ZHANG Li-min, PENG Juan-hua, WEI Man-man, DING Jian-nan, ZHOU Hong-bo. Bioleaching of chalcopyrite with Acidianus manzaensis YN25 under contact and non-contact conditions [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(10): 1981-1986.
[22] GU Guo-hua, SUN Xiao-jun, HU Ke-ting, LI Jian-hua, QIU Guan-zhou. Electrochemical oxidation behavior of pyrite bioleaching by Acidthiobacillus ferrooxidans [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(5): 1250-1254.
[23] RAWLINGS D E, SILVER S. Mining with microbes [J]. Biotechnology, 1995, 12: 773-778.
[24] YU Run-lan, ZHONG Dai-li, MIAO Lei, WU Fa-deng, QIU Guan-zhou, GU Guo-hua. Relationship and effect of redox potential, jarosites and extracellular polymeric substances in bioleaching chalcopyrite by Acidithiobacillus ferrooxidans [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(7): 1634-1640.
[25] FANG D, ZHOU L X. Effect of sludge dissolved organic matter on oxidation of ferrous iron and sulfur by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans [J]. Water, Air, Soil Pollut, 2006, 171: 81-94.
[26] PENG Juan-hua, ZHANG Rui-yong, ZHANG Qian, ZHANG Li-min, ZHOU Hong-bo. Screening and characterization of Acidiphilium sp. PJH and its role in bioleaching [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(6): 1443-1449.
[27] BACELAR-NICOLAU P, JOHNSON D B. Leaching of pyrite by acidophilic heterotrophic iron-oxidizing bacteria in pure and mixed cultures [J]. Appl Environ Microbiol, 1999, 65: 585-590.
[28] REZZA I, SALINAS E, ELORZA M, TOSETTI M S, DONATI E. Mechanisms involved in bioleaching of an aluminosilicate by heterotrophic microorganisms [J]. Process Biochem, 2001, 36: 495-500.
[29] JOHNSON D B. Importance of microbial ecology in the development of new mineral technologies [J]. Hydrometallurgy, 2001, 59: 147-157.
[30] BEOLCHINI F, DELL’ANNO A, PROPRIS L D, UBALDINI S, CERRONE F, DANOVARO R. Auto- and heterotrophic acidophilic bacteria enhance the bioremediation efficiency of sediments contaminated by heavy metals [J]. Chemosphere, 2009, 74: 1321-1326.
[31] JOHNSON D B, ROBERTO F F. Heterotrophic acidophiles and their roles in the bioleaching of sulfide minerals [C]//RAWLINGS D E. Biomining: Theory, Microbes and Industrial Processes. New York: Springer, 1997: 259-279.
[32] BOON M, HEIJNEN J J. Gas-liquid mass transfer phenomena in biooxidation experiments of sulphide minerals: A review of literature data [J]. Hydrometallurgy, 1998, 48: 187-204.
[33] GERICKE M, PINCHES A, van ROOYEN J V. Bioleaching of a chalcopyrite concentrate using an extremely thermophilic culture [J]. Int J Miner Process, 2001, 62: 243-255.
[34] AKCIL A, CIFTCI H, DEVECI H. Role and contribution of pure and mixed cultures of mesophiles in bioleaching of a pyritic chalcopyrite concentrate [J]. Miner Eng, 2007, 20: 310-318.
Acidiphilium cryptum对Acidithiobacillus ferrooxidans生物溶解磷矿的作用
肖春桥,池汝安,方玉娟
武汉工程大学 绿色化工过程教育部重点实验室,武汉 430073
摘 要:比较了嗜酸自养菌Acidithiobacillus ferrooxidans、嗜酸异养菌Acidiphilium cryptum、At. ferrooxidans和A. cryptum的混合菌在9K基体盐培养基中对黄铁矿的生物浸出以及磷矿的生物溶解。结果表明,虽然A. cryptum自身不能氧化黄铁矿和溶解磷矿,但能有效促进 At. ferrooxidans对黄铁矿的生物浸出以及磷矿的生物溶解。这种促进效应可通过A. cryptum促进At. ferrooxidans生物浸出黄铁矿体系中pH的降低以及氧化还原电位、总铁浓度和浮游细菌数目的升高的实验结果来证明。At. ferrooxidans和A. cryptum的混合菌液在30 °C条件下溶解磷矿时可最大程度地释放其中的可溶性磷。矿浆浓度大于3%时会给At. ferrooxidans和A. cryptum的混合菌液释放可溶性磷带来不利影响。在9K基体盐培养基中添加黄铁矿对At. ferrooxidans和A. cryptum的混合菌液溶解磷矿是很有必要的,且磷矿和黄铁矿的质量比为1:2或1:3时可溶性磷浸出率较高。
关键词:Acidiphilium cryptum;生物浸出;黄铁矿;磷矿;生物溶解; Acidithiobacillus ferrooxidans
(Edited by Xiang-qun LI)
Foundation item: Project (51004078) supported by the National Natural Science Foundation of China; Project (NCET-11-0965) supported by the Program for New Century Excellent Talents in University, China; Project (2012FFA101) supported by the Natural Science Foundation of Hubei Province, China; Project (IRT0974) supported by the Program for Changjiang Scholars and Innovative Research Team in University, China; Project (2011CB411901) supported by the National Basic Research Program of China
Corresponding author: Ru-an CHI; Tel: +86-27-87195682; E-mail: whjhx@yahoo.com.cn
DOI: 10.1016/S1003-6326(13)62711-9
Abstract: The bioleaching of pyrite and biosolubilization of rock phosphate (RP) in 9K basal salts medium were compared by the following strains of an autotrophic acidophilic bacterium, Acidithiobacillus ferrooxidans, a heterotrophic acidophilic bacterium, Acidiphilium cryptum, and mixed culture of At. ferrooxidans and A. cryptum. The results show that A. cryptum is effective in enhancing the bioleaching of pyrite and biosolubilization of RP in the presence of At. ferrooxidans, although it could not oxidize pyrite and solubilize RP by itself. This effect is demonstrated experimentally that A. cryptum enhances a decrease in pH and an increase in redox potential, concentration of total soluble iron and planktonic part bacterial number in the broth during pyrite bioleaching processes by At. ferrooxidans. The mixed culture of At. ferrooxidans and A. cryptum leads to the most extensive soluble phosphate released at 30 °C. Pulp density exceeding 3% is shown to adversely influence the release of soluble phosphate by the consortium of At. ferrooxidans and A. cryptum. It is essential to add pyrite to the 9K basal salts medium for the biosolubilization of RP by the mixed culture of At. ferrooxidans and A. cryptum, and the percentage of soluble phosphate released is the greatest when the mass ratio of RP to pyrite is 1:2 or 1:3.


