DOI:10.19476/j.ysxb.1004.0609.2019.07.15
Li/CFx电池高温储存性能和失效机理
马 苓1, 2, 3,钟学奇1, 2,桑 林2,丁 飞2,张硕卿1,刘兴江1, 2,徐 强1
(1. 天津大学 化工学院,天津 300072;
2. 中国电子科技集团公司第十八研究所,化学与物理电源重点实验室,天津 300384;
3. 天津市津南区安全生产监督管理局,天津 300350)
摘 要:
为了深入探索Li/CFx电池的高温储存性能及其容量衰减规律,本文分别研究不同储存温度和不同储存时间对Li/CFx电池放电性能的影响,并分析了电池性能下降的机理。结果表明,Li/CFx电池的自放电行为会随着储存温度的升高和储存时间的延长而变得越来越严重。EIS的分析结果表明,CFx阴极的电荷转移电阻会随着自放电的加剧而不断地增大。SEM、XRD和拉曼光谱等分析测试结果表明,Li/CFx电池经过高温储存后,CFx阴极表面上会形成一层自放电产物层,导致Li/CFx电池的放电容量和放电的电压平台逐渐地下降,电压滞后效应也愈发明显。相对于储存时间,Li/CFx的自放电行为对储存温度的升高更加敏感。
关键词:
文章编号:1004-0609(2019)-07-1466-08 中图分类号:TM912.4 文献标志码:A
随着移动通信、航空航天、交通运输及军事装备等领域的技术进步,各种高比能动力电池的开发也已成为国民经济发展的迫切需求[1-2]。由于金属锂具有质量轻、电极电位负的特点,以锂为负极的锂原电池的开发受到了极大的关注[3-4]。锂原电池主要包括锂-二氧化锰(Li/MnO2)、锂-二氧化硫(Li/SO2)、锂-亚硫酰氯(Li/SOCl2)和锂-氟化碳(Li/CFx)等电池体系[5-7]。同其它原电池相比,Li/CFx电池的理论比能量值最高(2180 W·h/kg),同时Li/CFx电池还具有安全性高、放电电压平稳、对环境友好等优点,特别适合作为无人或封闭环境中使用的仪表装备的动力电源。如心脏起搏器、导弹点火系统、无线电发报机、水下电子探测仪等,尤其是作为军事远程侦查及士兵随身携带的通讯电源,应用潜力巨大[8-9]。近年来,为了提高Li/CFx电池的放电性能,国内外很多学者主要围绕电池的倍率性能和电解液的高低温使用性能进行了改进和提高,开展了大量的研究工作[10-17]。这些改进工作主要分为以下两个方面:一是在提高CFx阴极电导率方面,包括新型阴极材料的开发[10],混合型阴极的设计[11],材料制备方法的改进[12],高导电集流体(导电添加剂)的加入[13],CFx阴极的表面改性[14]等;二是在提高电解液性能方面,包括低温电解液添加剂的开发[15],高温电解液添加剂的开发[16],新型固态电解液的开发[17]等。
Li/CFx电池的放电机理如下[18]:
阳极反应
xLi+xS→xLi+·S+xe (1)
阴极反应
CFx+xLi+·S+xe→C(Li+·S-F-)x (2)
总反应
xLi+xS+CFx→C(Li+·S-F-)x→C+xLiF+xS (3)
其中,反应式中的S代表电解液中与锂离子配位聚集的溶剂分子。
此外,Li/CFx电池的放电过程,还可以用一个由CFx内核和放电产物层(LiF+C)所构成的“核-壳”模型来加以粗略地描述[19],如图1所示。随着放电过程的连续进行,CFx内核的半径也在不断地减小,而由LiF晶粒和无定形碳所组成的放电产物层的厚度,则会不断地增大[20]。
作为理论比能量最高的一次化学电源,Li/CFx电池在实际使用过程中不可避免地需要储存和搁置,特别是在航空和军事等应用领域中[21]。在储存过程中,特别是高温条件下,Li/CFx电池的容量会发生不同程度的衰减,而且会随着外界温度的升高而进一步加剧[22]。因此,深入探索Li/CFx电池在高温条件下的储存性能,对高性能Li/CFx电池的设计和开发具有重要的意义。但到目前为止,尚没有发现有关Li/CFx电池的高温储存性能及自放电行为的文献报道。本文采用EIS、SEM、XRD、拉曼光谱等分析测试手段,系统地研究了Li/CFx电池经历不同储存温度和不同储存时间后的放电性能变化,分析了Li/CFx电池在放电过程中发生衰减的机理,阐明了高温储存温度和时间对Li/CFx电池自放电行为的影响规律,为今后制定Li/CFx电池的军用和民用储存标准,提供了科学依据。
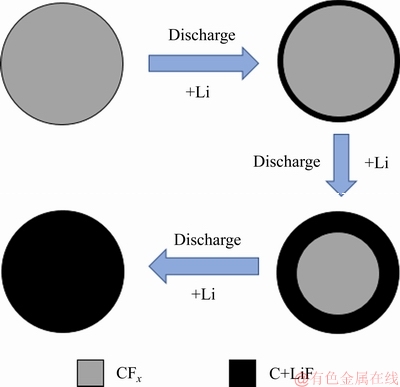
图1 描述Li/CFx电池放电过程的“核-壳”模型
Fig. 1 Schematic diagram of “core-shell” model of Li/CFx battery during discharge processes
1 实验
1.1 CFx电极的制备
将CFx、SP和水性胶等三种浆料原料,按照质量比90:5:5的比例混合均匀后,涂覆在纯铝箔上;在100 ℃温度下干燥后,经辊压、冲制成CR2430扣式电池所需的电极尺寸;于110 ℃温度下在真空干燥箱中静置24 h,烘干后备用。
1.2 扣式电池的组装
分别以烘干后的CFx电极和金属锂片为正、负极,以1 mol/L的LiBF4/DME+PC(DME与PC的体积比为2:1)溶液作为电解液,采用Celgard2400聚丙烯微孔膜作为隔膜,在充满高纯氩气的手套箱中,组装成CR2430型扣式电池,并在常温下静置4天。
1.3 电化学性能测试与材料表征
将所制备的扣式电池放置在恒温干燥箱中,分别于25、40、55和70 ℃等温度下进行存放。每隔5天分别从3个干燥箱中取出5只待测电池,等待测电池冷却至室温后,测量待测电池的开路电压及其电化学阻抗,然后进行恒电流放电测试。恒电流放电测试采用的设备是CT2001A型恒电流充放电仪(武汉蓝电公司),放电截止电压为1.5 V,测试的放电电流密度为86 mA/g。采用6360L型扫描电子显微镜(日本产)观察电极材料的表面形貌,用Bruker Advanc 型X射线衍射仪(日本产)分析电极材料的相组成,采用JY-T64000型激光拉曼光谱仪(HORIBA Jobin Yvon)对CFx阴极材料进行分析。采用电化学工作站(Solartron Instruments Model 1400/1470E)测量电池的电化学阻抗谱,正弦波信号的幅值为5 mV,测试频率范围为0.1~106 Hz。
2 结果与讨论
为了考察Li/CFx电池的高温自放电行为,本文分别采用固定存储时间和固定存储温度的方法,分类测试了Li/CFx电池在不同储存条件下的放电性能。图2所示为在固定储存时间(15天)的条件下,Li/CFx电池经过不同温度储存后的放电曲线图。由图2可以看出,Li/CFx电池储存前的放电比能量为825.2 mA·h/g(曲线A)。当Li/CFx电池分别在25、40、55和70 ℃的温度条件下储存15天后,Li/CFx电池的放电比容量发生了明显的衰减。不同温度下Li/CFx电池的剩余比容量分别为811.2 mA·h/g(曲线B)、799.2 mA·h/g(曲线C)、792.3 mA·h/g(曲线D)和630.8 mA·h/g(曲线E),对应的电池比容量衰减率分别为1.70%、3.15%、3.99%和23.56%。Li/CFx电池的放电平台也从储存前的2.52 V逐步降低到2.42 V。此外,储存后的Li/CFx电池放电的电压滞后现象,也会随着储存温度的升高而变得越来越明显。尤其是在70 ℃下,电池的初始放电电压平台已经下降到1.84 V。
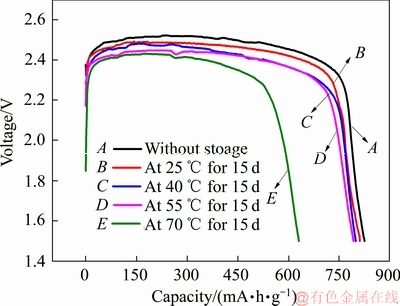
图2 Li/CFx电池在不同温度下储存15天后放电曲线
Fig. 2 Discharge curves of Li/CFx batteries at 86 mA/g after storage at different temperatures for 15 days
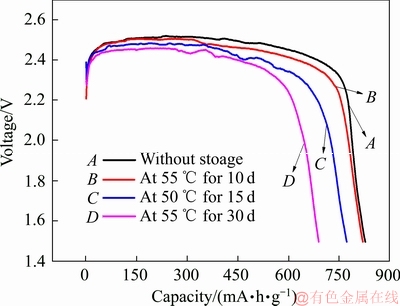
图3 Li/CFx电池在55 ℃下储存不同时间后的放电曲线
Fig. 3 Discharge curves of Li/CFx batteries at 86 mA/g after storage at 55 ℃ for different days
图3所示为在固定储存温度(55 ℃)的条件下,Li/CFx电池经过不同储存时间后的放电曲线图。由图3可以看出,当Li/CFx电池在55 ℃的温度条件下分别储存10、15和30天后,Li/CFx电池的放电比容量同样发生了明显的衰减。相对于储存前的Li/CFx电池(825.2 mA·h/g,曲线A),储存不同时间后的Li/CFx电池的剩余比容量分别为817.9 mA·h/g(曲线B)、792.3 mA·h/g(曲线C)和687.4 mA·h/g(曲线D),对应的电池比容量衰减率分别为0.88%,3.99%和16.70%。Li/CFx电池的放电平台也从储存前的2.52 V逐步降低到2.47 V。与图2相类似的是,储存后的Li/CFx电池放电的电压滞后现象,也会随着储存时间的延长而变得越来越严重。
根据化学电源的工作原理,储存温度和储存时间的增大,均会明显促进Li/CFx电池的自放电行为。由上述的Li/CFx电池的放电机理可知,Li/CFx电池的自放电属于一个自发的过程[23]。随着储存温度的升高和储存时间的延长,CFx阴极会不断地与金属锂负极发生自放电反应,电池的活性物质被不断地消耗掉,所以Li/CFx电池的放电比容量会逐渐减小,放电平台也变得越来越低。值得注意的是,相对于储存时间,Li/CFx电池的自放电行为对储存温度更加敏感。
图4所示为Li/CFx电池分别在25、40、55和70 ℃下储存了15天后的电化学阻抗(EIS)谱。从图4中可以发现,四条阻抗图谱均是由高频区的一个容抗弧和低频区的一条直线所构成。高频区容抗弧的直径代表了CFx阴极的电荷转移电阻(Rct),其中包括Li+在放电产物层中的迁移电阻和电极表面的电荷转移电阻。而低频区的直线则对应Li+扩散至CFx阴极晶格内部的Warburg阻抗[24]。从图4中的内嵌图中可以看出,Li/CFx电池储存前的Rct值约为33 Ω,而在25、40、55和70 ℃储存15天后的Li/CFx电池的Rct分别增大到73 Ω(曲线A)、101 Ω(曲线B)、489 Ω(曲线C)和1109 Ω(曲线D)。即当储存相同时间时,Li/CFx电池的Rct值会随着储存温度的升高而迅速增加。图5所示为Li/CFx电池在55 ℃温度下储存时,分别储存10、15和30天后的电化学阻抗图谱。如图5所示,Li/CFx电池的Rct分别约为165 Ω(曲线A)、398 Ω(曲线B)和696 Ω(曲线C)。即随着储存时间的延长,Li/CFx电池的Rct值也会随之增大。
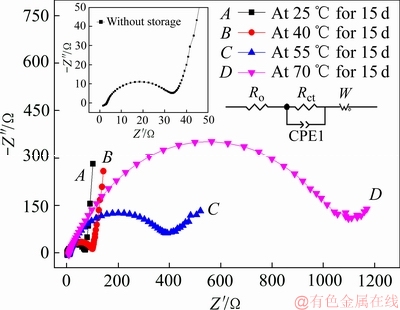
图4 Li/CFx电池在不同温度下储存15天后的电化学阻抗 图谱
Fig. 4 Impedance spectra of Li/CFx batteries after storage at different temperatures for 15 days
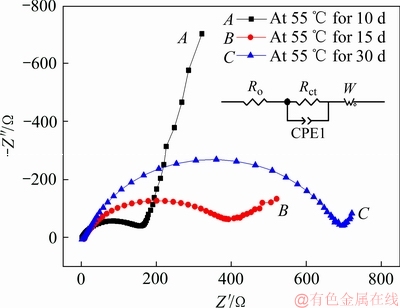
图5 Li/CFx电池在55 ℃下储存不同时间后的阻抗图谱
Fig. 5 Impedance spectra of Li/CFx batteries after storage at 55 ℃ for different days
上述EIS的测试结果表明,随着储存温度的升高和储存时间的延长,CFx阴极/电解液界面的电化学反应阻力会逐渐增加,即电荷转移电阻会不断地增大[25]。根据前述的Li/CFx电池放电过程的“核-壳”模型可知,当Li/CFx电池发生自放电时,CFx阴极表面会被一层放电产物层(C+LiF)所包覆。这层放电产物层会阻断电解液与CFx阴极的进一步接触,导致CFx阴极表面的反应电阻增大。随着自放电过程的不断进行,Li/CFx电池的放电产物层也会变得越来越致密,使得Li+离子迁移到CFx阴极内部的阻力不断地增大。因此,随着储存温度的升高和储存时间的延长,Li/CFx电池交流阻抗谱的电荷转移电阻(Rct)会变得越来越大,这个结论与上述Li/CFx电池的放电测试结果相符。
为了进一步分析Li/CFx电池经过高温储存后发生容量衰减的原因,把储存一定时间后的Li/CFx电池(未放电)进行拆解,观察CFx阴极材料表面的形貌变化,结果如图6所示。在未储存的Li/CFx电池的CFx阴极表面上(见图6(a)),片状的CFx晶体均匀地分布在导电剂和粘结剂的混合物中[26]。图6(b)~(e)显示了在不同储存温度下储存15天后的CFx阴极表面形貌。从图6(b)和(c)中可以发现,CFx阴极表面形貌基本上与图6(a)相似,表明Li/CFx电池的自放电程度还不太严重。而图6(d)和(e)则显示,CFx阴极表面上布满了细小的立方型LiF晶粒,表明随着储存时间的延长,Li/CFx电池的自放电行为会越来越严重。相对于经过外电路电流放电后的Li/CFx电池,CFx阴极表面上自放电形成的LiF晶粒的大小比较均匀, 这是由于Li/CFx电池自放电的电流密度较小且分布均匀的缘故[27]。图6(f)~(h)是在不同储存温度下储存15天后的Li/CFx电池的CFx阴极表面形貌。从这三张图片中可以看出,CFx阴极表面上同样充满了致密的LiF晶粒,表明经过高温的储存后,完整的放电产物层(LiF+C)已经在CFx阴极表面上形成。
为了进一步考察Li/CFx电池在高温下的自放电行为,对储存后拆解下来的CFx阴极表面进行了XRD测试分析。CFx阴极样品经过不同温度和不同时间储存后的XRD分析结果,如图7所示。从未储存的CFx阴极样品XRD分析曲线中可以发现,在13°和26.5°附近出现了CFx阴极材料的非晶态特征峰[28],表明此时Li/CFx电池的自放电现象并不严重,放电产物尚未完全覆盖CFx阴极材料的表面。而在65°和79°处则出现了两个LiF晶体特征峰,这是由于Li/CFx电池在常温下静置4天后所产生的轻微自放电所造成的。经过高温储存后,所有CFx阴极样品的XRD曲线上均出现了LiF晶体的特征峰(38°、45°、65°和79°),同时在上述XRD曲线中,13°处的非晶态特征峰几乎消失,表明自放电产物LiF已经完全覆盖在CFx内核的表面上。此外,随着储存温度的升高和储存时间的延长,CFx阴极材料表面上的LiF晶体的特征峰变得更加尖锐,说明放电产物LiF晶体的结晶度变得越来越高[29],表明Li/CFx电池的自放电反应经过高温储存后得到了明显的加速。
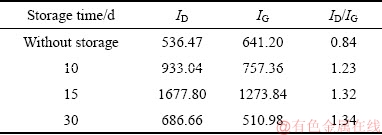
图8和9所示为Li/CFx电池在不同温度下储存不同时间后拆解下来的CFx阴极表面的拉曼光谱。由图8和9可见,两峰谱线强度的比值ID/IG可以反映出样品结晶的有序程度,即ID/IG的比值越小,说明样品的结晶越完整,有序化的程度也越高[30]。从图8和9中的谱线中可以发现,CFx阴极表面的拉曼光谱中均存在两个峰,即位于1356 cm-1附近的D峰和位于1582 cm-1附近的G峰[31]。这两个图中的谱线具有一个相似的特点,就是随着储存温度的升高或储存时间的延长,拉曼光谱的ID/IG比值呈现出逐渐增大的一种变化趋势。表1和2分别列出了图8和9中的ID/IG的比值。拉曼光谱ID/IG比值的这种变化趋势反映了不同CFx阴极表面结构的排列分布状况,可以用Li/CFx电池放电产物的“核-壳”模型来加以解释。在储存开始时,Li/CFx电池自放电产生的放电产物(LiF+C)较少,零散地分布在CFx阴极的表面上,所以其ID/IG比值较低。随着储存温度的升高或储存时间的延长,Li/CFx电池的自放电现象会逐渐加剧,分布在CFx阴极表面的放电产物数量也会变得越来越多,放电产物层也会变得越来越厚。由于膨胀过程和热应力的作用,放电产物层的有序程度会随着自放电的加剧而变得越差,造成CFx阴极表面的拉曼光谱的ID/IG比值会不断地升高。
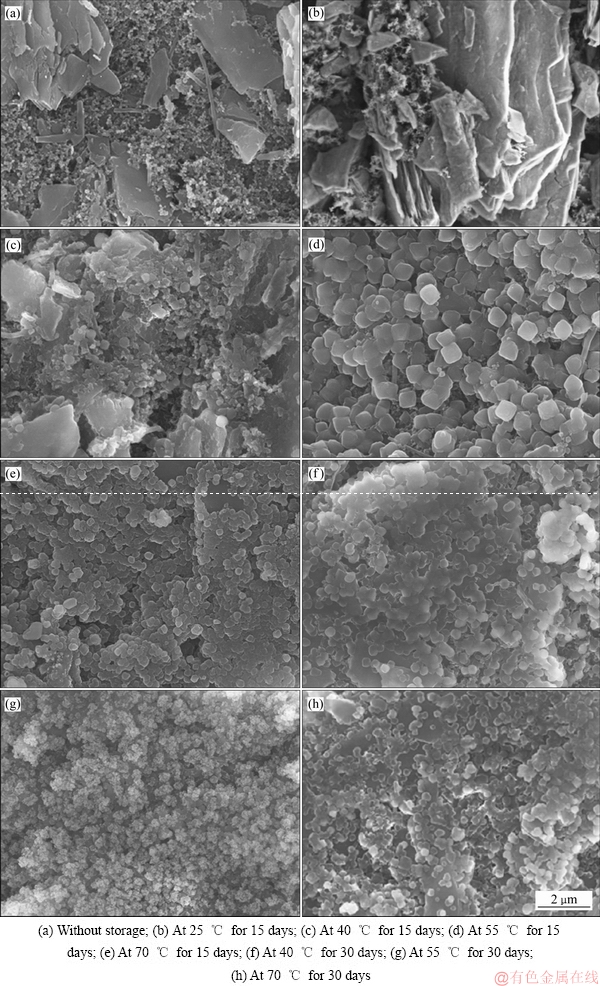
图6 CFx阴极的SEM像
Fig. 6 SEM images of CFx cathodes
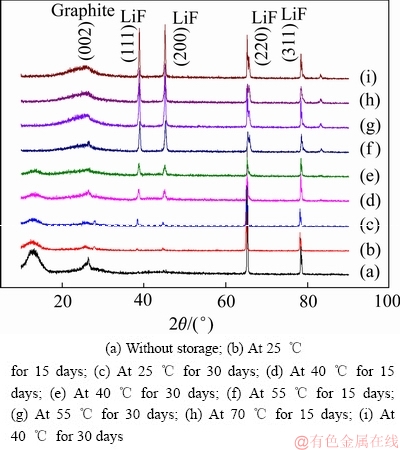
图7 CFx阴极在不同温度下储存不同时间后的XRD谱
Fig. 7 XRD patterns of CFx cathodes after storage at different temperatures for various days
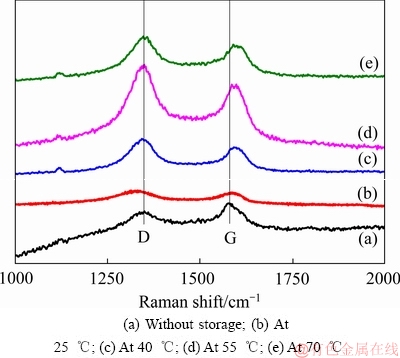
图8 CFx阴极在不同温度下储存15天后的Raman谱
Fig. 8 Raman spectra of CFx cathodes after storage at different temperatures for 15 days
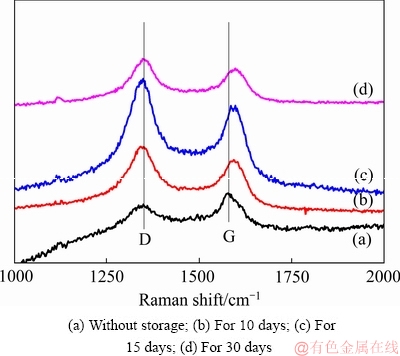
图9 CFx阴极在55 ℃下储存不同时间后的Raman谱
Fig. 9 Raman spectra of CFx cathodes after storage at 55 ℃for different days
表1 CFx阴极在不同温度下储存15天后Raman谱的ID/IG值
Table 1 ID/IG values in Raman patterns of CFx cathodes after storage at different temperatures for 15 days
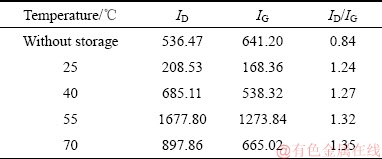
表2 CFx阴极在55 ℃下储存不同时间后Raman谱的ID/IG值
Table 2 ID/IG values in Raman patterns of CFx cathodes after storage at 55 ℃ for different days
3 结论
1) Li/CFx电池的自放电行为会随着储存温度的升高和储存时间的延长而变得越来越严重,CFx阴极的电荷转移电阻会随着自放电的加剧而不断增大。
2) 根据SEM、XRD和拉曼光谱等分析测试结果可知,Li/CFx电池经过高温储存后,CFx阴极表面会形成一层自放电产物层,导致Li/CFx电池的放电容量和放电的电压平台下降,电压滞后现象也愈发明显。
3) 相对于储存时间,Li/CFx的自放电行为对储存温度的升高更加敏感。
REFERENCES
[1] ARMAND M, TARASCON J M. Building better batteries[J]. Nature, 2008, 451(7179): 652.
[2] PENG S K, YAN S J, WANG N, WANG J X, CHENG X, WANG C, QIN X, DAI S L. Fluorinated graphene/sulfur hybrid cathode for high energy and high power density lithium primary batteries[J]. RSC Advances, 2018, 8(23): 12701-12707.
[3] KRAUSE F C, JONES J P, JONES S C, PASALIC J, BILLINGS K J, WEST W C, SMART M C, BUGGA R V, BRANDON E J, DESTEPHEN M. High specific energy lithium primary batteries as power sources for deep space exploration[J]. Journal of the Electrochemical Society, 2018, 165(10): A2312-A2320.
[4] ZHONG G M, CHEN H X, HUANG X K, YUE X J, LU C Z. High-power-density, high-energy-density fluorinated graphene for primary lithium batteries[J]. Frontiers in Chemistry, 2018, 6(50): 1-9. doi: 10.3389/fchem.2018.00050
[5] CHENG S J, YUAN ZZ, YE X P, ZHANG F Y, LIU J C. Empirical prediction model for Li/SOCl2 cells based on the accelerated degradation test[J]. Microelectronics Reliability, 2015, 55(1): 101-106.
[6] DOSE W M, DONNE S W. Optimising heat treatment environment and atmosphere of electrolytic manganese dioxide for primary Li/MnO2 batteries[J]. Journal of Power Sources, 2014, 247: 852-857.
[7] AHMAD Y, DUBOIS M, GUERIN K, HAMWI A, FLAHAUT E. High energy density of primary lithium batteries working with sub-fluorinated few walled carbon nanotubes cathode[J]. Journal of Alloys and Compounds, 2017, 726: 852-859.
[8] DAI Y, FANG Y, CAI S D, WU L J, YANG W J, Yan H, XIE J Y, ZHENG J C, TAKEUCHIE, ZHU Y M. Surface modified pinecone shaped hierarchical structure fluorinated mesocarbon microbeads for ultrafast discharge and improved electrochemical performances[J]. Journal of the Electrochemical Society, 2017, 164(2): A1-A7.
[9] SMITH P H, JR R B S, WATERMAN K G, MYRON L J. Development and analysis of a lithium carbon monofluoride battery-lithium ion capacitor hybrid system for high pulse-power applications[J]. Journal of Power Sources, 2016, 327: 495-506.
[10] ZHU Y L, ZHANG L J, ZHAO H H, FU Y. Significantly improved electrochemical performance of CFx promoted by SiO2 modification for primary lithium batteries[J]. Journal of Materials Chemistry A, 2017, 5(2): 796-803.
[11] LI Y, FENG W. The tunable electrochemical performances of carbon fluorides/manganese dioxide hybrid cathodes by their arrangements[J]. Journal of Power Sources, 2015, 274: 1292-1299.
[12] SUN C B, FENG YY, LI Y, QIN C Q, ZHANG Q Q, FENG W. Solvothermally exfoliated fluorographene for high- performance lithium primary batteries[J]. Nanoscale, 2014, 6(5): 2634-2641.
[13] YIN X D, LI Y, FENGY Y, FENG W. Polythiophene/graphite fluoride composites cathode for high power and energy densities lithium primary batteries[J]. Synthetic Metals, 2016, 220: 560-566.
[14] LI L, ZHU L, PAN Y, LEI W X, MA Z S, LI Z Z, CHENG J J, ZHOU J. Integrated polyaniline-coated CFx cathode materials with enhanced electrochemical capabilities for Li/CFx primary battery[J]. International Journal of Electrochemical Science, 2016, 11: 6838-6847.
[15] IGNATOVA A A, YARMOLENKO O V, TULIBAEVA G Z, SHESTAKOV A F, FATEEV S A. Influence of 15-crown-5 additive to a liquid electrolyte on the performance of Li/CFx—Systems at temperatures up to -50 ℃[J]. Journal of Power Sources, 2016, 309: 116-121.
[16] 张懋慧, 杨炜婧, 解晶莹, 袁黎明. 氟化磷酸酯对锂/氟化碳电池放电性能的影响[J]. 电池, 2015, 45(2): 65-67.
ZHANG Mao-hui, YANG Wei-jing, XIE Jing-ying, YUAN Li-ming. Effect of fluorination phosphate ester on discharge performance of Li/CFx battery[J]. Battery Bimonthly, 2015, 45(2): 65-67.
[17] RANGASAMY E, LI J C, SAHU G, DUDNEY N, LIANG C D. Pushing the theoretical limit of Li-CFx batteries: A tale of bifunctional electrolyte[J]. Journal of the American Chemical Society, 2014, 136(19): 6874-6877.
[18] AHMAD Y, DUBOIS M, GUERIN K, HAMWI A, ZHANG W. Pushing the theoretical limit of Li-CFx batteries using fluorinated nanostructured carbon nanodiscs[J]. Carbon, 2015, 94: 1061-1070.
[19] ZHANG S S, FOSTER D, WOLFENSTINE J, READ J. Electrochemical characteristic and discharge mechanism of a primary Li/CFx cell[J]. Journal of Power Sources, 2009, 187(1): 233-237.
[20] ZHU Y L, ZHANG L J, ZHAO HH, FU Y. Significantly improved electrochemical performance of CFx promoted by SiO2 modification for primary lithium batteries[J]. Journal of Materials Chemistry A, 2017, 5(2): 796-803.
[21] 阳晓霞, 段 征, 金晶龙, 王 燕, 王希文. 高比能氟化碳材料及Li/CFx电池的特性研究[J]. 电源技术, 2018, 42(8): 1161-1162.
YANG Xiao-xia, DUAN Zheng, JIN Jing-long, WANG Yan, WANG Xi-wen. Performance research of polycarbon mono-fluoride and Li/CFx cell [J]. Chinese Journal of Power Sources, 2018, 42(8): 1161-1162.
[22] 卢立丽, 王松蕊. 锂氟化碳电池放电热效应的模拟研究[J]. 电源技术, 2016, 40(5): 1098-1102.
LU Li-li, WANG Song-rui. Studies on thermal effects during discharging of 1ithium carbon fluoride cells by simulation[J]. Chinese Journal of Power Sources, 2016, 40(5): 1098-1102.
[23] 乔学荣, 米 娟, 刘志伟. 锂氟化碳电池安全性研究[J]. 电源技术, 2017, 41(8): 1127-1129.
QIAO Xue-rong, MI Juan, LIU Zhi-wei. Safety research of Li/CFx battery[J]. Chinese Journal of Power Sources, 2017, 41(8): 1127-1129.
[24] LI Y, CHEN Y F, FENG W, DING F, LIU X J. The improved discharge performance of Li/CFx batteries by using multi- walled carbon nanotubes as conductive additive[J]. Journal of Power Sources, 2011, 196(4): 2246-2250.
[25] ZHANG S S, FOSTER D, READ J. Carbothermal treatment for the improved discharge performance of primary Li/CFx battery[J]. Journal of Power Sources, 2009, 191(2): 648-652.
[26] READ J, COLLINS E, PIEKARSKI B, ZHANG S. LiF formation and cathode swelling in the Li/CFx battery[J]. Journal of the Electrochemical Society, 2011, 158(5): A504-A510.
[27] PANG C K, DING F, SUN W B, LIU J Q, HAO M M, WANG Y, LIU X J, XU Q. A novel dimethyl sulfoxide/1, 3-dioxolane based electrolyte for lithium/carbon fluorides batteries with a high discharge voltage plateau[J]. Electrochimica Acta, 2015, 174: 230-237.
[28] REDDY M A, BREITUNG B, FICHTNER M. Improving the energy density and power density of CFx by mechanical milling: A primary lithium battery electrode[J]. ACS Applied Materials & Interfaces, 2013, 5(21): 11207-11211.
[29] Rodriguez M A, KEENAN M R, NAGASUBRAMANIAM G. In situ X-ray diffraction analysis of (CFx)n batteries: signal extraction by multivariate analysis[J]. Journal of Applied Crystallography, 2007, 40(6): 1097-1104.
[30] 汪刘应, 徐 卓, 华绍春,刘安明, 郭 秦, 刘 顾. 不同管径CNTs/Al2O3-TiO2复合吸波涂层的拉曼光谱特征及吸波性能研究[J]. 无机材料学报, 2013, 28(2): 136-140.
WANG Liu-ying, XU Zhuo, HUA Shao-chun, LIU An-ming, GUO Qin, LIU Gu. Raman spectroscopy and microwave absorbing properties of CNTs/Al O3-TiO2 composite absorbing coatings with different diameters[J]. Journal of Inorganic Materials, 2013, 28(2): 136-140.
[31] AHMAD Y, DUBOIS M, GUERIN K, HAMWI A, FLAHAUT E. High energy density of primary lithium batteries working with sub-fluorinated few walled carbon nanotubes cathode[J]. Journal of Alloys and Compounds, 2017, 726: 852-859.
Storage performances and failure mechanisms of Li-CFx batteries at high temperature
MA Ling1, 2, 3, ZHONG Xue-qi1, 2, SANG Lin2, DING Fei2, ZHANG Shuo-qing1, LIU Xing-jiang1, 2, XU Qiang1
(1. School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China;
2. National Laboratory of Science and Technology on Power Sources, Tianjin Institute of Power Sources, Tianjin 300384, China;
3. Jinnan Administration of Work Safety, Tianjin 300350, China)
Abstract: In order to investigate the storage performances and failure mechanisms, the self-discharge behaviors of both the Li/CFx batteries stored at different temperatures and for different days were studied in detail. The discharge results show that the capacity of Li/CFx batteries fades rapidly with increasing the storage temperature or storage time. The testing results of EIS, SEM XRD and Raman spectroscopy indicate that a layer of self-discharge products may be produced on the surface of CFx cathode after storage at high temperature. The self-discharge products can decrease both the Rct and discharge voltage platform, and also promote the voltage hysteresis of Li/CFx cells obviously. Compared with the storage time, the self-discharge behavior of Li/CFx batteries is more sensitive to the increase of storage temperature.
Key words: Li-battery; carbon fluoride; storage at high temperature; loss of capacity; self-discharge
Foundation item: Project(17JCYBJC20900) supported by the General Project of Natural Science Foundation of Tianjin, China
Received date: 2018-04-15; Accepted date: 2019-04-02
Corresponding author: XU Qiang; Tel: +86-13702108410; E-mail: xuqiang@tju.edu.cn
Juxtaposed first author: MA Ling, ZHONG Xue-qi
(编辑 何学锋)
基金项目:天津市自然科学基金面上项目(17JCYBJC20900)
收稿日期:2018-04-15;修订日期:2019-04-02
通信作者:徐 强,副教授,博士;电话:13702108410;E-mail:xuqiang@tju.edu.cn;并列第一作者:马 苓,钟学奇
摘 要:为了深入探索Li/CFx电池的高温储存性能及其容量衰减规律,本文分别研究不同储存温度和不同储存时间对Li/CFx电池放电性能的影响,并分析了电池性能下降的机理。结果表明,Li/CFx电池的自放电行为会随着储存温度的升高和储存时间的延长而变得越来越严重。EIS的分析结果表明,CFx阴极的电荷转移电阻会随着自放电的加剧而不断地增大。SEM、XRD和拉曼光谱等分析测试结果表明,Li/CFx电池经过高温储存后,CFx阴极表面上会形成一层自放电产物层,导致Li/CFx电池的放电容量和放电的电压平台逐渐地下降,电压滞后效应也愈发明显。相对于储存时间,Li/CFx的自放电行为对储存温度的升高更加敏感。
[1] ARMAND M, TARASCON J M. Building better batteries[J]. Nature, 2008, 451(7179): 652.
[16] 张懋慧, 杨炜婧, 解晶莹, 袁黎明. 氟化磷酸酯对锂/氟化碳电池放电性能的影响[J]. 电池, 2015, 45(2): 65-67.
[21] 阳晓霞, 段 征, 金晶龙, 王 燕, 王希文. 高比能氟化碳材料及Li/CFx电池的特性研究[J]. 电源技术, 2018, 42(8): 1161-1162.
[22] 卢立丽, 王松蕊. 锂氟化碳电池放电热效应的模拟研究[J]. 电源技术, 2016, 40(5): 1098-1102.
[23] 乔学荣, 米 娟, 刘志伟. 锂氟化碳电池安全性研究[J]. 电源技术, 2017, 41(8): 1127-1129.


