
J. Cent. South Univ. (2021) 28: 361-369
DOI: https://doi.org/10.1007/s11771-021-4608-y
Green large-scale production of N/O-dual doping hard carbon derived from bagasse as high-performance anodes for sodium-ion batteries
WANG Jin(王金)1, LI Yu-shan(李育珊)1, LIU Peng(刘鹏)1, WANG Feng(王凤)1, YAO Qing-rong(姚青荣)1,
ZOU Yong-jin(邹勇进)1, ZHOU Huai-ying(周怀营)1, BALOGUN M.-Sadeeq2, 3, DENG Jian-qiu(邓健秋)1, 2
1. School of Materials Science and Engineering, Guilin University of Electronic Technology, Guilin 541004, China;
2. Guangxi Key Laboratory of Information Materials, Guilin University of Electronic Technology, Guilin 541004, China;
3. School of Materials Science and Engineering, Hunan University, Changsha 410012, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract:
Sodium-ion batteries are considered as a promising candidate for lithium-ion batteries due to abundant sodium resources and similar intercalation chemistry. Hard carbon derived from biomass with the virtue of abundance and renewability is a cost-effective anode material. Herein, hard carbon is derived from renewable bagasse through a simple two-step method combining mechanical ball milling with carbonization. The hard carbon electrodes exhibit superior electrochemical performance with a high reversible capacity of 315 mA·h/g. Furthermore, the initial capacity of the full cell, HC//NaMn0.4Ni0.4Ti0.1Mg0.1O2, is 253 mA·h/g and its capacity retention rate is 77% after 80 cycles, which further verifies its practical application. The simple and low-cost preparation process, as well as excellent electrochemical properties, demonstrates that hard carbon derived from bagasse is a promising anode for sodium-ion batteries.
Key words:
anode; hard carbon; sodium-ion batteries; cycling stability; full cell;
Cite this article as:
WANG Jin, LI Yu-shan, LIU Peng, WANG Feng, YAO Qing-rong, ZOU Yong-jin, ZHOU Huai-ying, BALOGUN M.-Sadeeq, DENG Jian-qiu. Green large-scale production of N/O-dual doping hard carbon derived from bagasse as high-performance anodes for sodium-ion batteries [J]. Journal of Central South University, 2021, 28(2): 361-369.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-021-4608-y1 Introduction
Lithium-ion batteries (LIBs) have been diffusely used due to their high specific capacity and long-life [1-3]. However, the uneven distribution and lack of Li in the lithosphere, as well as the high cost, significantly limit the applications of LIBs in larger-scale electrical energy storage (EES). Therefore, researchers have made much effort to develop new battery technologies to satisfy the needs of extensive EES. Sodium-ion batteries (SIBs) are ideal alternatives for massive EES applications as a result of similar intercalation chemistry to lithium and the natural abundance of sodium resources [4-6].
In recent years, cathode materials of SIBs have been studied systematically, including transition layered oxides [7, 8], polyanionic compounds [9, 10], and prussian blue analogues [11, 12]. The key point of the commercialization of SIBs is low-cost as well as high-performance anodes. In this respect, metal and alloys [13, 14], oxides [15, 16], and carbon materials [17, 18] used for SIBs seem to be more advantageous. For metal and alloy anode materials, the larger volume expansion will cause the structural collapse, then result in the rapid capacity fading [19]. The oxides have low capacity and high polarization, possibly due to the sluggish diffusion kinetics of Na-ions, limiting their practical application in SIBs [20]. The primary drawback of the graphite, one of the carbonaceous materials, is the capacity too low to suit for SIBs [21]. Unlike the former, the biomass-derived hard carbon (HC) for SIBs has been explored prosperously for its abundance, low cost, nontoxicity, cycling stability, and durability [22-24]. For example, peat moss [25], cotton [26], banana peels [27], kelp [28], shaddock peels [29], pistachios [30], and mangosteen shells [31] have been used to prepare HC as the anode materials.
Bagasse is an abundant agricultural abandoned byproduct. So far, bagasse is usually burned, causing a waste of resources and air pollution. The high content of hemicellulose, lignin, and cellulose in bagasse makes it become a suitable carbon source to prepare carbon electrodes used for high-performance energy storage systems, such as LIBs and supercapacitors [32, 33]. So far, there are only a few reports about the application possibility of bagasse-derived carbon for SIBs anode. Herein, we propose to use bagasse, an agricultural waste, as a precursor with a simple and efficient route to prepare HC materials for SIBs anode. The carbon materials carbonized at 900 °C show excellent sodium storage performance. To further prove the practical application, rechargeable sodium-ion full cells, HC//NaNi0.4Mn0.4Ti0.1Cu0.1O2, were fabricated, offering excellent electrochemical performance. This work provides an approach of large-scale production of HC materials for the high- performance anode in SIBs by utilizing rich agricultural waste via a green synthesis procedure.
2 Experimental
2.1 Preparation and characterization of HC
In the preparation procedure of HC materials, the bagasse was firstly washed and dried at 80 °C, and then crushed by ball milling (400 r/min, 6 h). The HC materials were obtained by carbonizing the bagasse at 800, 900, 1000 °C in a tube furnace for 2 h in Ar atmosphere, and named as HC-800, HC-900, HC-1000, respectively.
The crystal structure of HC materials was characterized by a PIXcel3D powder diffractometer with CuKα radiation (1.5406 A). Scanning electron microscope (SEM, FEI QUANTA FEG 450) and transmission electron microscope (TEM, FEI Talos F200X) were used to inspect the microstructure of HC materials. Raman spectra were obtained from a Horiba LabRAM HR800 with 514 nm radiation at laser radiation of 0.4 mW in the range of 100-2000 cm-1.
2.2 Electrochemical measurements
A typical preparation of electrodes and cells has been reported before [34]. The electrolyte used was a mixture of 1 mol/L NaClO4 in ethylene carbonate (EC) and diethyl carbonate (DEC) (1:1 in volume). Cyclic voltammetry (CV) at 0.1 mV/s within the range of 0.01-3 V and galvanostatic intermittent titration technique (GITT) with the pulse current of 25 mA/g for 0.5 h with an interval of 1 h were implemented on a Modulab electrochemical workstation (Solartron analytical). And electrochemical impedance spectroscopy (EIS) measurements were conducted on a CHI660D electrochemical workstation.
3 Results and discussion
3.1 Structural characterization
X-ray diffraction (XRD) was conducted to study the structure of HC materials. As shown in Figure 1(a), two broad peaks around 24° and 43°, correspond to (002) and (101) planes of the crystallographic, indicating that the obtained HC materials are amorphous or disordered carbons. According to the diffraction peak position corresponding to (002) crystal plane, the layer spacing is calculated to be about 0.377 nm using the Bragg equation (2dsinθ=nλ), which facilitates the insertion/de-insertion of Na-ion. The two separate characteristic peaks from Raman spectra in Figure 1(b) at 1343 cm-1 (D-band) and 1586 cm-1 (G-band), correspond to the disordered carbon and graphitic carbon, respectively. The integrated intensity ratio (IG/ID) is 0.59, 0.66, and 0.69 for the HC-800, HC-900, and HC-1000, respectively, indicating that the ordered degree of HC materials increases with increasing carbonization temperature [17]. The element composition and surface characteristics of the HC materials were investigated by XPS analysis, as displayed in Figure S1 (Supporting information) and Table 1. Elements C and O are dominated with a small amount of N and Si in all three HC materials. Heteroatoms O and N are observed for the high-resolution XPS spectra of C 1 s (Figure 1(c)) for three HC, which could provide extra reaction sites for reversible Na-ion insertion/ extraction and improve the electronic conductivity [35, 36].
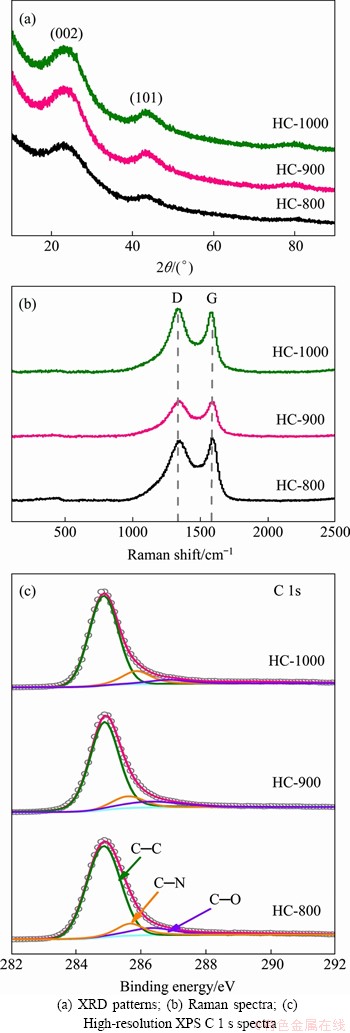
Figure 1 Structure of HC materials prepared at various temperatures:
Table 1 Structural parameters of HC materials
Furthermore, the morphology of HC materials was also investigated using SEM and TEM. The SEM images of HC-800, HC-900, and HC-1000 are displayed in Figures 2(a)-(c), respectively. It found that all of these HC materials have irregular micro/ nano-sized particles. And HC particles become larger and denser under higher calcination temperature, relating to the degree of graphitization. The TEM images of HC-900 are shown in Figures 2(d)-(f). The structure of HC-900 is orderly short-range and disorderly long-range, suggesting that few graphitized carbons dispersed in amorphous carbons. The dispersive diffraction ring from the SAED pattern further proves the disordered microstructure of the HC-900. The SEM and TEM results well agree with the XRD data and Raman spectra.
3.2 Electrochemical performance of HC electrode
The comparisons of the electrochemical properties of the HC electrodes are shown in Figure 3(a) and (b). Their first discharge capacity is 269.1, 589.3, and 319.7 mA·h/g for HC-800, HC-900, and HC-1000, respectively. The slope region and platform region of the discharge curves are the process of the adsorption and intercalation of sodium ions, respectively. The higher discharge capacity of HC-900 may be due to its proper layer spacing and morphology, which is more helpful to the storage of Na-ions. Besides, these HC materials show good cycle performances at 0.2C. From Figure 3(b), the capacity retention after 200 cycles is 84.8%, 85.8%, and 96.8% of the 2nd discharge capacity for HC-800, HC-900, and HC-1000, respectively. In the first cycle, due to the solid electrolyte interface (SEI) layer and the adsorption of inactive, the initial coulomb efficiency (CE) of the HC-900 electrode is only 55%. The charge/discharge curves of HC-900 at different cycles also prove it. In Figure 3(c), the discharge curves overlapped except for the first one mean good cycle stability. Furthermore, the long-cycle performance of HC-900 is investigated as well. At a rate of 10C, the first discharge capacity is 288.7 mA·h/g (Figure 3(d)) with an initial CE of 63%. Due to the polarization of the electrode at 10C, the slope region of the discharge curve shifts to the right, and the platform area is not obvious. After 800 cycles, the capacity remains at 61% of the 2nd discharge capacity (Figure 3(e)).

Figure 2 SEM images of HC-800 (a), HC-900 (b) and HC-1000 (c); TEM (d, e) and SAED (f) images of HC-900
3.3 Kinetic study
The initial three CV curves of the HC-900 electrode are shown in Figure 4(a). There are two sharp peaks at 0.1 V representing Na-ion insertion and extraction. Besides, the irreversible peaks at 0.72 V and 0.38 V from the first cycle suggest the irreversible loss of Na-ion during the construction of the SEI layer. Besides, the 2nd and 3rd cycle curves are coincided perfectly, which demonstrates that the construction of SEI mainly takes place in the first cycle and reversible Na-ion insertion/ de-insertion reactions in the subsequent cycles.
The Na-ions diffusion coefficient (DNa+) of HC electrodes was estimated by GITT method (Figure S2). As the voltage is linear with τ1/2 from a single GITT curve (Figures 4(b) and (c)), DNa+ can be obtained based on Fick’s second law equation [37, 38]. As shown in Figure 4(d), the value of DNa+ is about 10-14 cm2/s. During the discharge/charge process, the DNa+ decreases gradually except for abnormal fluctuations occurring at 0.1-0.3 V, which means that the reaction happens at this moment. These results are well consistent with the adsorption-intercalation-pore filling model [17]. Initially, sodium ions are only adsorbed on the surface of the HC electrode. At 0.3 V, the DNa+ increases due to the sodium intercalation reaction. With the reaction going on, the surface-active sites of the HC electrode gradually saturate, and the DNa+ decreases. Finally, sodium ions enter the closed internal micropores of HC materials, increasing DNa+ again.
The Nyquist plots (Figure S3) of the fresh and cycled HC-900 electrode were measured in the frequency range of 100-0.01 Hz with perturbation amplitude of 5 mV. The Rct values of the fresh and cycled electrodes are 347 and 304 Ω, respectively, which are larger than those of the other HC materials reported [24, 28]. This may be due to the larger surface area of hard carbon materials prepared via ball-milling and carbonization.
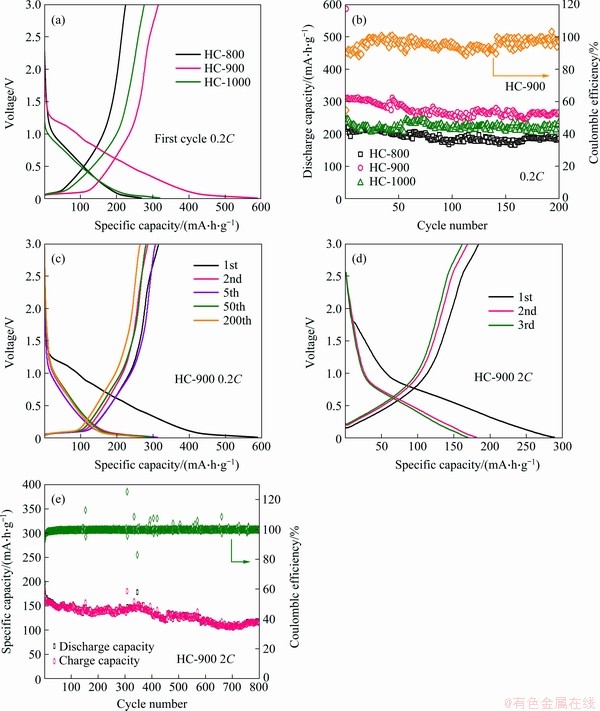
Figure 3 (a) Charge-discharge profiles at the first cycle for HC electrodes at 0.2C; (b) Cycling performances of HC electrodes at 0.2C; (c) Charge-discharge profiles of HC-900 at 0.2C; (d) The initial three charge-discharge profiles of HC-900 at 2C; (e) Cycling performance of HC-900 at 2C
3.4 Electrochemical performance of full cell
The sodium ion full cells fabricated with the O3-type NaNi0.4Cu0.1Mn0.4Ti0.1O2 as the cathode and the HC-900 as the anode was used to demonstrate the applicability in practice. The homemade cathode materials obtained based on our previous report [34], delivering a high capacity, appropriate voltage window, and good capacity retention, can well match with the HC anode. The full cells are tested at 0.1C (25 mA/g) from 1.0 to 4.0 V. Compared with the half cell (Figure 3(c)), the full cell delivers a lower initial capacity of 253 mA·h/g with a CE of 50% and higher operating potential of 3.3 V (Figure 5(a)). After 80 cycles, capacity retention of the full cell is 77% with a reversible specific capacity of 194.4 mA·h/g (Figure 5(b)). These acceptable properties of our full cells suggest that these HC materials have satisfactory application prospects in SIBs.
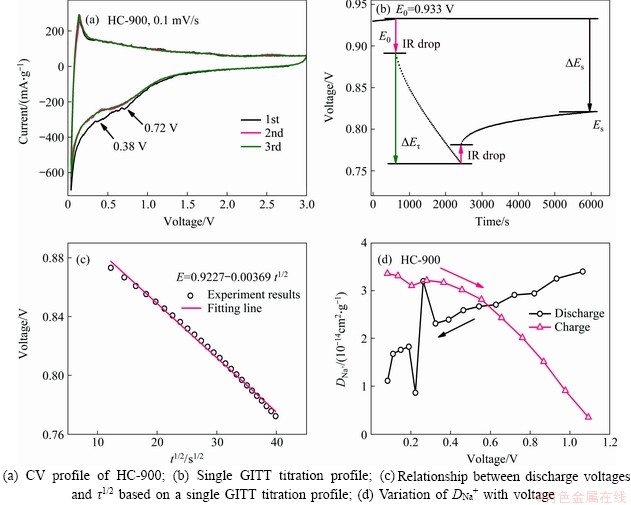
Figure 4 Kinetic study of HC-900 electrode:
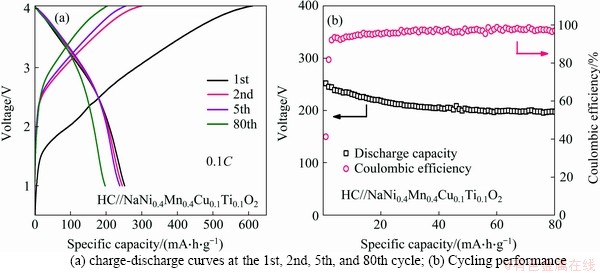
Figure 5 Electrochemical performance of HC//NaNi0.4Cu0.1Mn0.4Ti0.1O2 full cells at 0.1C:
4 Conclusions
The resource-abundant, low-cost, and highly reversible HC materials were prepared from bagasse waste through facile ball-milling and carbonization method. The sample HC-900 displays a high reversible capacity (330 mA·h/g) at 50 mA/g with a capacity retention of 98% after 200 cycles. Also, its sodium-ion storage and transport kinetic behaviors have been explored by the CV and GITT technologies. Further to prove its practical application, the fabricated HC//NaNi0.4Cu0.1Mn0.4Ti0.1O2 full cells still have a reversible specific capacity of 194.4 mA·h/g and capacity retention of 77% after 80 cycles. All these results demonstrate that hard carbon materials prepared from bagasse are a promising candidate anode for SIBs.
Contributors
The initial ideas and goals were developed by DENG Jian-qiu, ZHOU Huai-ying and ZOU Yong-jin. WANG Jin and LI Yu-shan conducted experimental tests. LIU Peng, WANG Feng and YAO Qing-rong analyzed the experimental data. YAO Qing-rong, ZOU Yong-jin and ZHOU Huai-ying revised the figures and provided theoretical guidance. LIU Peng, DENG Jian-qiu, and BALOGUN M.-Sadeeq wrote, revised, and polished the manuscript. All authors replied to reviewers’ comments and revised the final version.
Conflict of interest
WANG Jin, LI Yu-shan, LIU Peng, WANG Feng, YAO Qing-rong, ZOU Yong-jin, ZHOU Huai-ying, BALOGUN M.-Sadeeq and DENG Jian-qiu declare that they have no conflict of interest.
References
[1] CHENG Xin-bing, ZHANG Rui, ZHAO Chen-zi, ZHANG Qiang. Toward safe lithium metal anode in rechargeable batteries: A review [J]. Chemical Reviews, 2017, 117(15): 10403-10473. DOI: 10.1021/acs.chemrev. 7b00115.
[2] LARCHER D, TARASCON J M. Towards greener and more sustainable batteries for electrical energy storage [J]. Nature Chemistry, 2015, 7(1): 19-29. DOI: 10.1038/nchem. 2085.
[3] TANG Jia-liang, ETACHERI V, POL V G. Wild fungus derived carbon fibers and hybrids as anodes for lithium-ion batteries [J]. ACS Sustainable Chemistry & Engineering, 2016, 4(5): 2624-2631. DOI: 10.1021/ acssuschemeng.6b00114.
[4] NAYAK P K, YANG L T, BREHM W, ADELHELM P. From lithium-ion to sodium-ion batteries: Advantages, challenges, and surprises [J]. Angewandte Chemie-International Edition, 2018, 57(1): 102-120. DOI: 10.1002/ anie.201703772.
[5] ALVIN S, YOON D, CHANDRA C, CAHYADI H S, PARK J, CHANG W, CHUNG K Y, KIM J. Revealing sodium ion storage mechanism in hard carbon [J]. Carbon, 2019, 145: 67-81. DOI: 10.1016/j.carbon.2018.12.112.
[6] WU Fei-xiang, ZHAO Cheng-long, CHEN Shuang-qiang, LU Ya-xiang, HOU Yang-long, HU Yong-Sheng, MAIER J, YU Yan. Multi-electron reaction materials for sodium-based batteries [J]. Materials Today, 2018, 21(9): 960-973. DOI: 10.1016/j.mattod.2018.03.004.
[7] RADIN M D, THOMAS J C, VAN D V A. Order-disorder versus displacive transitions in Jahn-Teller active layered materials [J]. Physical Review Materials, 2020, 4(4): 043601. DOI: 10.1103/PhysRevMaterials.4.043601.
[8] PAHARI D, PURAVANKARA S. On controlling the P2-O2 phase transition by optimal Ti-substitution on Ni-site in P2-type Na0.67Ni0.33Mn0.67O2 (NNMO) cathode for Na-ion batteries [J]. Journal of Power Sources, 2020, 455: 227957. DOI: 10.1016/j.jpowsour.2020.227957.
[9] WANG Jing-yang, WANG Yan, SEO D H, SHI Tan, CHEN Shou-ping, TIAN Yao-sen, KIM H, CEDER G. A high-energy NASICON-type cathode material for Na-ion batteries [J]. Advanced Energy Materials, 2020, 10(10): 1903968. DOI: 10.1002/Aenm.201903968.
[10] WANG Fu-xiang, LIU Shan-shan, JIANG Qi-ke, FENG Kai, YANG Xin, LI Xing-cun, ZHANG Hong-zhang, XIA Ming-jun, ZHANG Hua-min. K2Fe3(SO4)3(OH)2(H2O)2: A new high-performance hydroxysulfate cathode material for alkali metal ion batteries [J]. Journal of Power Sources, 2020, 452: 227835. DOI: 10.1016/j.jpowsour.2020.227835.
[11] ZHOU Ai-jun, CHENG Wei-jie, WANG Wei, ZHAO Qiang, XIE Jian, ZHANG Wu-xing, GAO Hong-cai, XUE Lei-gang, LI Jing-ze. Hexacyanoferrate-type prussian blue analogs: Principles and advances toward high-performance sodium and potassium ion batteries [J]. Advanced Energy Materials, 2020, 11(2): 202000943. DOI: 10.1002/Aenm.202000943.
[12] LIU Qian-nan, HU Zhe, CHEN Ming-zhe, ZOU Chao, JIN Hui-le, WANG Shun, CHOU Shu-lei, LIU Yong, DOU Shi-xue. The cathode choice for commercialization of sodium-ion batteries: Layered transition metal oxides versus prussian blue analogs [J]. Advanced Functional Materials, 2020, 30(14): 201909530. DOI: 10.1002/Adfm.201909530.
[13] PFEIFER K, ARNOLD S, BUDAK O, LUO Xian-lin, PRESSER V, EHRENBERG H, DSOKE S. Choosing the right carbon additive is of vital importance for high-performance Sb-based Na-ion batteries [J]. Journal of Materials Chemistry A, 2020, 8(12): 6092-6104. DOI: 10.1039/d0ta00254b.
[14] LI Hai-xia, WANG Ji-wei, JIAO Li-fang, TAO Zhan-liang, LIANG Jing. Spherical nano-SnSb/C composite as a high-performance anode material for sodium ion batteries [J]. Acta Physico-Chimica Sinica, 2020, 36(5): 1904017. DOI: 10.3866/Pku.Whxb201904017.
[15] CHAI Yu-jun, DU Yong-heng, LI Ling, WANG Ning. Dual metal oxides interconnected by carbon nanotubes for high-capacity Li- and Na-ion batteries [J]. Nanotechnology, 2020, 31(21): 215402. DOI: 10.1088/1361-6528/ab7049.
[16] SHE Liao-na, ZHANG Feng, JIA Cong-ying, KANG Li-ping, LI Qi, HE Xue-xia, SUN Jie, LEI Zhi-bin, LIU Zong-huai. Electrospun Nb2O5 nanorods/microporous multichannel carbon nanofiber film anode for Na+ ion capacitors with good performance [J]. J Colloid Interface Sci, 2020, 573: 1-10. DOI: 10.1016/j.jcis.2020.03.122.
[17] XIAO Bi-wei, ROJO T, LI Xiao-lin. Hard carbon as sodium-ion battery anodes: Progress and challenges [J]. Chem Sus Chem, 2019, 12(1): 133-144. DOI: 10.1002/cssc. 201801879.
[18] WU Feng, ZHANG Ming-hao, BAI Ying, WANG Xin-ran, DONG Rui-qi, WU Chuan. Lotus seedpod-derived hard carbon with hierarchical porous structure as stable anode for sodium-ion batteries [J]. ACS Applied Materials & Interfaces, 2019, 11(13): 12554-12561. DOI: 10.1021/acsami.9b01419.
[19] YANG Hai, CHEN Lin-wei, HE Fu-xiang, ZHANG Jia-qing, FENG Yue-zhan, ZHAO Lu-kang, WANG Bin, HE Li-xin, ZHANG Qiao-bao, YU Yan. Optimizing the void size of yolk-shell Bi@Void@C nanospheres for high-power-density sodium-ion batteries [J]. Nano Letters, 2020, 20(1): 758-767. DOI: 10.1021/acs.nanolett.9b04829.
[20] LI Yu-zhu, WANG Huan-wen, WANG Li-bin, MAO Zhi-fei, WANG Rui, HE Bei-bei, GONG Yan-sheng, HU Xian-luo. Mesopore-induced ultrafast Na+-storage in T-Nb2O5/carbon nanofiber films toward flexible high-power Na-ion capacitors [J]. Small, 2019, 15(9): 1804539. DOI: 10.1002/smll.201804539.
[21] LIU Yuan-yue, MERINOV B V, GODDARD W A III. Origin of low sodium capacity in graphite and generally weak substrate binding of Na and Mg among alkali and alkaline earth metals [J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(14): 3735-3739. DOI: 10.1073/pnas.1602473113.
[22] ZHU You-yu, CHEN Ming-ming, LI Qi, YUAN Chao, WANG Cheng-yang. A porous biomass-derived anode for high-performance sodium-ion batteries [J]. Carbon, 2018, 129: 695-701.
[23] LIU Ting, LI Xi-fei. Biomass-derived nanostructured porous carbons for sodium ion batteries: A review [J]. Materials Technology, 2018, 34(4): 232-245. DOI: 10.1016/j.carbon. 2017.12.103.
[24] HAO Jian, WANG Yan-xia, CHI Cai-xia, WANG Jing, GUO Qing-jie, YANG Yu, LI Yao, LIU Xiao-xu, ZHAO Jiu-peng. Enhanced storage capability by biomass-derived porous carbon for lithium-ion and sodium-ion battery anodes [J]. Sustainable Energy & Fuels, 2018, 2(10): 2358-2365. DOI: 10.1039/c8se00353j.
[25] DING Jia, WANG Huan-lei, LI Zhi, KOHANDEHGHAN A, CUI Kai, XU Zhan-wei, ZAHIRI B, TAN Xue-hai, LOTFABAD E M, OLSEN B C, MITLIN D. Carbon nanosheet frameworks derived from peat moss as high performance sodium ion battery anodes [J]. ACS Nano, 2013, 7(12): 11004-11015. DOI: 10.1021/nn404640c.
[26] LI Yun-ming, HU Yong-sheng, TITIRICI M M, CHEN Li-quan, HUANG Xue-jie. Hard carbon microtubes made from renewable cotton as high-performance anode material for sodium-ion batteries [J]. Advanced Energy Materials, 2016, 6(18): 1600659. DOI: 10.1002/aenm.201600659.
[27] LOTFABAD E M, DING J, CUI K, KOHANDEHGHAN A, KALISVAART W P, HAZELTON M, MITLIN D. High-density sodium and lithium ion battery anodes from banana peels [J]. ACS Nano, 2014, 8(7): 7115-7129. DOI: 10.1021/nn502045y.
[28] WANG Peng-zi, ZHU Xiao-shu, WANG Qiao-qiao, XU Xin, ZHOU Xiao-si, BAO Jian-chun. Kelp-derived hard carbons as advanced anode materials for sodium-ion batteries [J]. Journal of Materials Chemistry A, 2017, 5(12): 5761-5769. DOI: 10.1039/c7ta00639j.
[29] LI Rui-zi, HUANG Jian-feng, LI Jia-yin, CAO Li-yun, ZHONG Xin-zi, YU Ai-min, LU Guo-xing. Nitrogen-doped porous hard carbons derived from shaddock peel for high-capacity lithium-ion battery anodes [J]. Journal of Electroanalytical Chemistry, 2020, 862: 114044. DOI: 10.1016/j.jelechem.2020.114044.
[30] BENITEZ A, MORALES J, CABALLERO A. Pistachio shell-derived carbon activated with phosphoric acid: A more efficient procedure to improve the performance of Li-S batteries [J]. Nanomaterials (Basel), 2020, 10(5): 840. DOI: 10.3390/nano10050840.
[31] XUE Ming-zhe, CHEN Chen, TAN Yan, REN Zhi-wei, LI Bing, ZHANG Cun-man. Mangosteen peel-derived porous carbon: Synthesis and its application in the sulfur cathode for lithium sulfur battery [J]. Journal of Materials Science, 2018, 53(15): 11062-11077. DOI: 10.1007/s10853-018-2370-9.
[32] LUAN Rui-ying, XU Da, PAN Hui, ZHU Cheng-ling, WANG Da-wei, MENG Xin, LI Yao, IMTIAZ M, ZHU Shen-min, MA Jun. High electrochemical cycling performance through accurately inheriting hierarchical porous structure from bagasse [J]. Journal of Energy Storage, 2019, 22: 60-67. DOI: 10.1016/j.est.2019.01.021.
[33] LI Yu-zhu, WANG Huan-wen, HUANG Bao-jun, WANG Li-bin, WANG Rui, HE Bei-bei, GONG Yan-sheng, HU Xian-luo. Mo2C-induced solid-phase synthesis of ultrathin MoS2 nanosheet arrays on bagasse-derived porous carbon frameworks for high-energy hybrid sodium-ion capacitors [J]. Journal of Materials Chemistry A, 2018, 6(30): 14742-14751. DOI: 10.1039/c8ta04597f.
[34] WANG Jin, ZHOU Zhao-fu, LI Yu-shan, LI Meng, WANG Feng, YAO Qing-rong, WANG Zhong-min, ZHOU Huai-ying, DENG Jian-qiu. High-rate performance O3-NaNi0.4Mn0.4Cu0.1Ti0.1O2 as a cathode for sodium ion batteries [J]. Journal of Alloys and Compounds, 2019, 792: 1054-1060. DOI: 10.1016/j.jallcom.2019.04.053.
[35] DU Lei-lei, WU Wei, LUO Chao, XU Dong-wei, GUO Han-yu, WANG Ruo, ZHANG Tian, WANG Jun, DENG Yong-hong. Lignin-derived nitrogen-doped porous carbon as a high-rate anode material for sodium ion batteries [J]. Journal of the Electrochemical Society, 2019, 166(2): A423-A428. DOI: 10.1149/2.1361902jes.
[36] ZENG Guang, ZHOU Bao-long, YI Luo-cai, LI Hao, HU Xiang, LI Yan. Green and facile fabrication of hierarchical N-doped porous carbon from water hyacinths for high performance lithium/sodium ion batteries [J]. Sustainable Energy & Fuels, 2018, 2(4): 855-861. DOI: 10.1039/c7se00517b.
[37] ZHANG Yan-jia, LI Xue, DONG Peng, WU Gang, XIAO Jie, ZENG Xiao-yuan, ZHANG Ying-jie, SUN Xue-liang. Honeycomb-like hard carbon derived from pine pollen as high-performance anode material for sodium-ion batteries [J]. ACS Applied Materials & Interfaces, 2018, 10(49): 42796-42803. DOI: 10.1021/acsami.8b13160.
[38] WANG Jing, YAN Lei, REN Qing-juan, FAN Lin-lin, ZHANG Fu-ming, SHI Zhi-qiang. Facile hydrothermal treatment route of reed straw-derived hard carbon for high performance sodium ion battery [J]. Electrochimica Acta, 2018, 291: 188-196. DOI: 10.1016/j.electacta.2018.08.136.
(Edited by ZHENG Yu-tong)
中文导读
绿色大规模制备甘蔗渣衍生的N/O双掺杂硬碳用作高性能钠离子电池负极
摘要:得益于丰富的钠资源和类似的插层化学性质,钠离子电池被认为是一种很有前途的锂离子电池替代者。生物质硬碳具有来源丰富、可再生的优点,是一种高性价比的阳极材料。本文采用简单两步法,机械球磨和炭化,将可再生甘蔗渣制备成硬碳材料。这种硬碳电极具有较高的可逆容量(315 mA·h/g),表现出优秀的电化学性能。此外,在全电池HC//NaMn0.4Ni0.4Ti0.1Mg0.1O2中的初始容量达253 mA·h/g,80次循环后容量仍有77%,进一步验证了其实际应用价值。简单、低成本的制备工艺以及优异的电化学性能表明,甘蔗渣硬碳是一种很有前途的钠离子电池负极材料。
关键词:阳极;硬碳;钠离子电池;循环稳定性;全电池
Foundation item: Projects(51661009, 51761007) supported by the National Natural Science Foundation of China; Projects (2019GXNSFDA245014, 2016GXNSFGA380001) supported by the Natural Science Foundation of Guangxi Province, China; Projects(2019AC20164, 2019AC20053) supported by the Science and Technology Base and Talent Special Project of Guangxi Province, China
Received date: 2020-07-15; Accepted date: 2020-10-28
Corresponding author: LIU Peng, PhD; Tel: +86-13207521833; E-mail: liupeng@guet.edu.cn; ORCID: https://orcid.org/0000-0003- 0492-4473; DENG Jian-qiu, PhD, Professor; E-mail: jqdeng@guet.edu.cn, ORCID:https://orcid.org/0000- 0002-8628-9719; BALOGUN M.-Sadeeq, PhD, Professor; E-mail: balogun@hnu.edu.cn; ORCID: https://orcid.org/0000-0001-5313-7893
Abstract: Sodium-ion batteries are considered as a promising candidate for lithium-ion batteries due to abundant sodium resources and similar intercalation chemistry. Hard carbon derived from biomass with the virtue of abundance and renewability is a cost-effective anode material. Herein, hard carbon is derived from renewable bagasse through a simple two-step method combining mechanical ball milling with carbonization. The hard carbon electrodes exhibit superior electrochemical performance with a high reversible capacity of 315 mA·h/g. Furthermore, the initial capacity of the full cell, HC//NaMn0.4Ni0.4Ti0.1Mg0.1O2, is 253 mA·h/g and its capacity retention rate is 77% after 80 cycles, which further verifies its practical application. The simple and low-cost preparation process, as well as excellent electrochemical properties, demonstrates that hard carbon derived from bagasse is a promising anode for sodium-ion batteries.


