Trans. Nonferrous Met. Soc. China 23(2013) 3691-3696
Synthesis of Cu2O/reduced graphene oxide composites as anode materials for lithium ion batteries
Guo-chun YAN, Xin-hai LI, Zhi-xing WANG, Hua-jun GUO,Qian ZHANG, Wen-jie PENG
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 20 January 2013; accepted 12 September 2013
Abstract:
A facile way was used to synthesize Cu2O/reduced graphene oxide (rGO) composites with octahedron-like morphology in aqueous solution without any surfactant. TEM images of the obtained Cu2O/rGOs reveal that the Cu2O particles and rGO distribute hierarchically and the primary Cu2O particles are encapsulated well in the graphene nanosheets. The electrochemical performance of Cu2O/rGOs is enhanced compared with bare Cu2O when they are employed as anode materials for lithium ion batteries. The Cu2O/rGO composites maintain a reversible capacity of 348.4 mA×h/g after 50 cycles at a current density of 100 mA/g. In addition, the composites retain 305.8 mA×h/g after 60 cycles at various current densities of 50, 100, 200, 400 and 800 mA/g.
Key words:
cuprous oxide; reduced graphene oxide; anode material;
1 Introduction
Currently, transition metal oxides, such as Co3O4, Fe2O3, Mn3O4 and CuO, have attracted great research interests as alternative anode materials for lithium ion batteries because of their low cost and relatively high specific capacity compared with graphite materials [1-8]. However, these conversion-based materials have inherently inferior cycling performance due to their severe volume expansion and contraction during the discharge and charge process. Cuprous oxide (Cu2O) is also investigated as an anode material for lithium ion batteries, while it suffers from the same drawback of other transition metal oxides. Previous studies have confirmed that different particle size and morphology play a significant role in determining the electrochemical performance of Cu2O crystal [9-12].
Graphene was confirmed an excellent supporting material as it owns high surface area, superior electrical conductivity and flexibility [13,14]. XU et al [15] fabricated cubic-like cuprous oxide/graphene by two steps in absolute ethanol. Besides, it has been reported that Cu2O/reduced graphene oxide (rGO) composites were synthesized by one-pot hydrothermal treatment or by high intensity ultrasound assisting [16-19]. However, these methods are relatively complicated and are difficult to scale up. Moreover, graphene can disperse in water directly without any assistance of dispersing agent [20]. In order to buffer the volume change and alleviate the aggregation of graphene during the cycling process, constructing Cu2O/rGO composites is efficient to enhance the cycling performance and rate capability of Cu2O.
In this work, a facile way to synthesize Cu2O/rGO composites with octahedron-like morphology composites is represented. The composites are prepared by the reduction of GO and 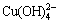 simultaneously using hydrazine as reducer in aqueous solution under a mild temperature and constant pressure without any surfactant.
simultaneously using hydrazine as reducer in aqueous solution under a mild temperature and constant pressure without any surfactant.
2 Experimental
2.1 Preparation of Cu2O and Cu2O/ rGO
Graphene oxide (GO) was synthesized from natural graphite by modified Hummer’s method [21]. To prepare Cu2O/rGO, 200 mL aqueous solution of CuSO4·5H2O (2.5 g) was added into round-bottomed flask, followed by 200 mL aqueous solution of NaOH (1.6 g) under magnetic stirring. After that, 100 mL GO (1 mg/mL) aqueous that was dispersed by sonication for 1 h was mixed in. Once the temperature of the mixed solution reached 50 °C, 3 mL hydrazine solution (1.5 mol/L) was added into it. After 2 h, the brown solid products were collected by filtration, washed with deionized water and ethanol three times, respectively, and dried in a vacuum oven at 80 °C for 12 h. The bare Cu2O was prepared by the same process without adding GO solution.
2.2 Materials characterization
The crystal structure of the products was determined by X-ray diffraction (XRD, Cu Kα radiation, Rint-2000, Rigaku). The morphology of the products was observed by scanning electron microscopy (SEM, Sirion200) and transmission electron microscopy (TEM, Tecnai G12). Fourier transform infrared (FT-IR) spectra were recorded by a Nicolet AVATAR 360 FTIR spectrometer. The content of graphene was determined by a carbon sulfur analyzer (HCS-140, Shanghai Dekai).
2.3 Electrochemical measurement
Electrochemical performance was measured in CR2025 coin-type cell with a Neware galvanostatic charge-discharge system. The anode electrodes were prepared by coating slurries that were made up of Cu2O or Cu2O/rGO (80% in mass fraction) with acetylene black (10% in mass fraction) and poly(vinylidene fluoride) (10% in mass fraction) as a binder dissolved in N-methyl pyrrolidinone solution on a copper foil. Lithium foil was used as a counter electrode. A polypropylene micro-porous film was employed as the separator. The electrolyte was a mixture of 1 mol/L LiPF6 in a mixture of ethylene carbonate (EC), ethyl methyl carbonate (EMC) and dimethyl carbonate (DMC) (1:1:1, v/v/v). The batteries were cycled galvanostatically between 0.001 V and 3.0 V at room temperature. Cyclic voltammetry (CV) test was carried out in a CHI600d electrochemical workstation at a scanning rate of 0.1 mV/s from 0 to 3.0 V. Electrochemical impedance spectroscopy (EIS) measurements were conducted with a CHI600d potentiostat over a frequency range from 100 kHz to 10 mHz. A three-electrode cell in a discharged- state was used for EIS test, where lithium foils were used as both reference and counter electrode.
3 Results and discussion
3.1 Materials characterization
To hinder the aggregation between the electronegative functional groups of GO and electropositive cupric ions caused by electrostatic attraction, the NaOH solution was added into CuSO4 solution according to the stoichiometric ratio to form the coordination compound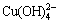 . Therefore, the GO and
. Therefore, the GO and 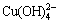 were dispersed homogeneously in aqueous [22]. They were reduced simultaneously once the hydrazine solution was added, and the Cu2O/rGO composites were prepared when the amount of hydrazine was appropriate. Despite our synthetic approach is different from the method of previous studies to synthesize metal oxides/rGO, in which the metal ions were added into the GO suspension and coordinated with carboxyl groups of GO [2,3,15-19,23,24], the newly formed Cu2O particle will be embedded well in the reduced graphene oxide either.
were dispersed homogeneously in aqueous [22]. They were reduced simultaneously once the hydrazine solution was added, and the Cu2O/rGO composites were prepared when the amount of hydrazine was appropriate. Despite our synthetic approach is different from the method of previous studies to synthesize metal oxides/rGO, in which the metal ions were added into the GO suspension and coordinated with carboxyl groups of GO [2,3,15-19,23,24], the newly formed Cu2O particle will be embedded well in the reduced graphene oxide either.
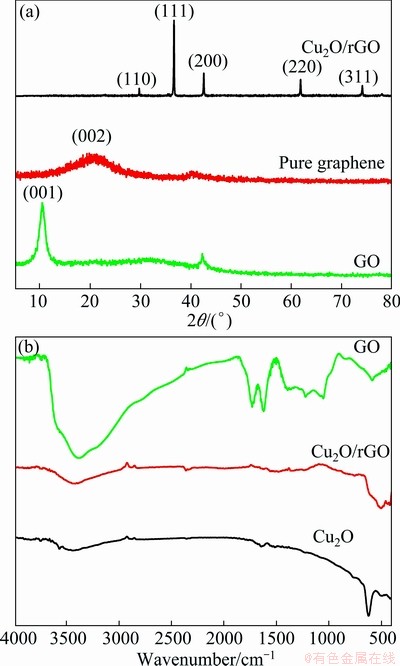
Fig. 1 XRD patterns of Cu2O/rGO, pure graphene and GO (a) and FTIR spectra of GO, Cu2O/rGO and Cu2O (b)
Figure 1(a) shows the X-ray diffraction patterns of Cu2O/rGO composites, pure graphene and GO. The GO shows a sharp (001) diffraction peak, which indicates that the interplanar spacing of GO is 0.840 nm. In addition, the pure graphene displays a broad (002) diffraction peak, which implies the interplanar spacing of rGO is 0.359 nm. Consequently, the decreased interplanar spacing demonstrates that the amount of GO reduces. The Bragg peaks of the Cu2O are clearly distinguishable, and all peaks can be exclusively indexed to the cubic phase Cu2O (JCPDS file No.05—0667), indicating the good crystalline nature of the Cu2O particle. It should be noted that no diffraction peaks for graphite could be observed, which suggests no further agglomeration of few layers of rGO in Cu2O/rGO composites. The graphene content in the composites is 5.455% that was determined by a carbon sulfur analyzer, which means the constituent of Cu2O in the composites is 94.545%. FTIR spectra of GO, Cu2O, and Cu2O/rGO are illustrated in Fig. 1(b). GO displays plentiful oxygen-containing groups, and the band around 3400 cm-1 arises from stretching vibration of O—H. The band at 1720 cm-1 reflects the C=O vibration of —COOH located at the edge of GO, and the peaks around 1367 cm-1, 1225 cm-1 and 1056 cm-1 can be attributed to the C—O groups on the surface of GO. While for Cu2O, the peak around 622 cm-1 is ascribed to Cu—O vibrating, and the relatively weak peaks at 3428 cm-1 are caused by the hydroxy of absorbed water inevitably. As for Cu2O/rGO, the Cu—O vibration shifts to 506 cm-1, suggesting that the interaction between graphene and Cu2O affects the vibration of Cu—O bonds.
The scanning electron microscopy images shown in Figs. 2(a) and (b) represent the typical Cu2O and Cu2O/rGO crystals synthesized, respectively. The Cu2O crystals with octahedron morphology are clearly seen with sizes ranging from 0.5 to 1 μm. However, the characteristic of octahedron morphology in Cu2O/rGO crystals is not as evident as Cu2O. This may be caused by the existence of graphene that affects the growth of Cu2O crystals. In addition, rGO sheets in Cu2O/rGO enwrap the Cu2O crystals and the size of Cu2O/rGO is around 400 nm, which can be clearly seen in Fig. 2(c). The high magnification TEM image of the edge in Fig. 2(d) reveals that the Cu2O particles and rGO distribute hierarchically.
3.2 Electrochemical properties
In order to evaluate the electrochemical performance of bare Cu2O and Cu2O/rGO, galvanostatic discharge-charge experiments were carried out. The 1st, 5th, 10th discharge-charge curves at a constant current density of 50 mA/g are illustrated in Figs. 3(a) and (b), in which the potential range is settled from 0.001 to 3.0 V, and the inset in Fig. 3(a) is the 1st discharge-charge curve of rGO. In the first cycle, the bare Cu2O shows a discharge and charge capacity of 667.7 mA×h/g and 330.8 mA×h/g respectively, and the initial coulombic efficiency is as low as 49.5%. In comparison, the Cu2O/rGO composites deliver a discharge and charge capacity of 771.7 and 567.3 mA×h/g respectively, which corresponds to 73.6% of the coulombic efficiency. The increased capacity (104 mA×h/g) is attributed to the addition of rGO because the rGO can delivery 1556.8 mA×h/g in the first discharge as shown in the inset of Fig. 3(a). Moreover, the reason why the initial coulombic efficiency rises in Cu2O/rGO composites is that the rGO makes the conversion reaction of Cu2O proceed easier. Furthermore, for bare Cu2O, the reversible capacity drops to 105.8 mA×h/g, whereas Cu2O/rGO composites still exhibit 478.6 mA×h/g after 10 cycles.
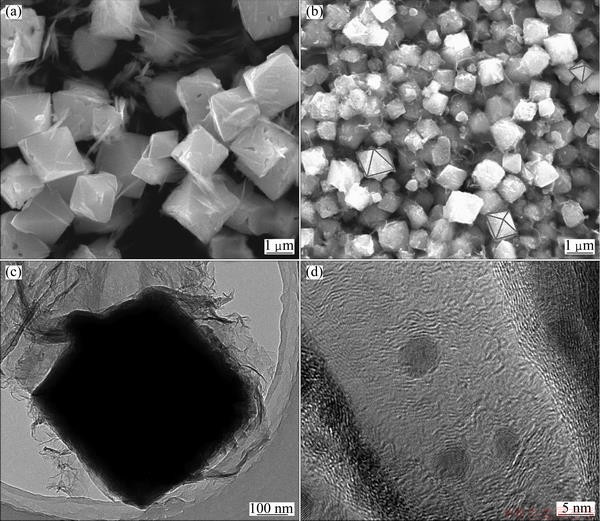
Fig. 2 SEM images of Cu2O (a) and Cu2O/rGO (b), TEM image of Cu2O/rGO (c) and HRTEM image of Cu2O/rGO in edge (d)
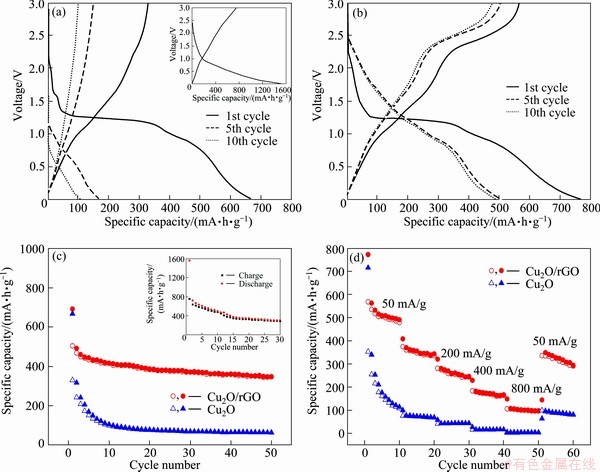
Fig. 3 Discharge and charge curves of bare Cu2O at 50 mA/g (inset shows the first discharge and charge curves of rGO at 50 mA/g) (a), Cu2O/rGO at 50 mA/g (b), cycling performance at 50 mA/g (inset shows cycling performance of rGO at 50 mA/g (c) and rate capability at various current densities (d)
Figure 3(c) demonstrates the cycling performance of bare Cu2O and Cu2O/rGO at a current density of 50 mA/g. As for bare Cu2O, the reversible capacity drops to 64.1 mA×h/g after 50 cycles while Cu2O/rGO still retains 348.4 mA×h/g, which demonstrates that the cycling capability is improved significantly. The poor capacity retention ability of bare Cu2O should be caused by the large volume exchange during the cycling process. In addition, the reversible capacity of bare rGO remains 301.6 mA×h/g after 30 cycles because rGO is prone to stack during the cycling process [14]. Figure 3(d) represents the rate capability of bare Cu2O and Cu2O/rGO from current densities of 50 to 800 mA/g after 10 cycles at each current density. After 60 cycles at various current densities, the Cu2O/rGO composites maintain a reversible capacity of 305.8 mA×h/g. Even at a current density of 800 mA/g, the reversible capacity of Cu2O/rGO composites is higher than that of bare Cu2O at a current density of 50 mA/g. These electrochemical data indicate that a little amount of graphene (5.455%) has a beneficial effect on the cycling performance and rate capability. This can be ascribed to two main reasons. The first is that the addition of rGO reduces the size of Cu2O, which is considered an effective way to improve the electrochemical performance of Cu2O as anode material for lithium ion battery. The other is that the superior flexibility of rGO is beneficial to buffering the huge volume exchange of Cu2O during cycling process.
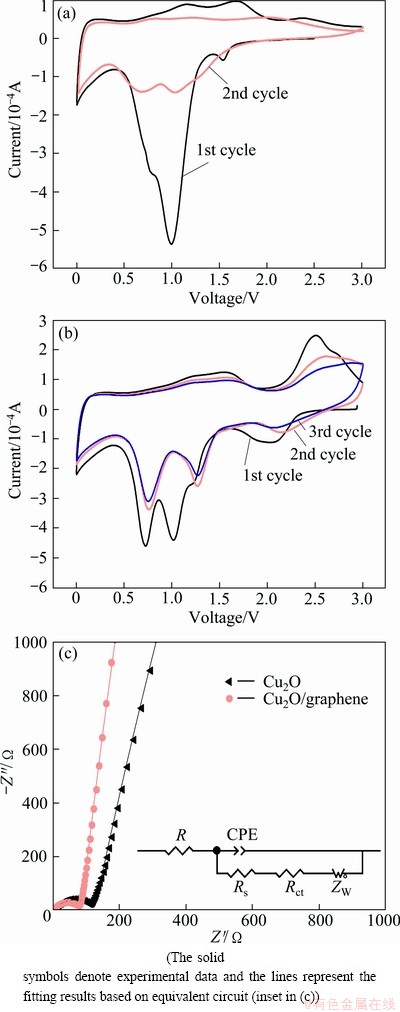
Fig. 4 Cyclic voltammograms of bare Cu2O with scan rate of 0.1 mV/s between 0 and 3.0 V (vs Li/Li+) (a), Cu2O/rGO (b), and Nyquist plots of bare Cu2O and Cu2O/rGO (c)
To further investigate the electrochemical performance, CV experiments were conducted. The cyclic voltammetric curves of bare Cu2O and Cu2O/rGO between 0 and 3 V at a scanning rate of 0.1 mV/s are shown in Figs. 4(a) and (b), respectively. For bare Cu2O sample in the first cycle, there is a well-defined peak ranging from 1.5 to 0.5 V, and a broad peak in the range of 0.4-0 V is observed during the cathodic process. The former peak can be attributed to the reduction of Cu2O to Cu and formation of Li2O, and the latter can be ascribed to the formation of a solid electrolyte interface (SEI) film [9]. As for the anodic process, two peaks in a range of 1.0-1.8 V are due to the decomposition of the SEI film, and the peak around 2.4 V corresponds to the conversion of Cu to Cu2O and the decomposition of Li2O. In the second cycle, the cathodic peak around 1.0 V in the first cycle is separated to two cathodic peaks. This owes to the decreased particle size of Cu2O, which leads to the potential of the conversion reaction between Cu2O and Li+ increasing [10]. Compared with bare Cu2O, there is a cathodic peak positioned at about 2.0 V, which indicates that Li+ inserts into graphene and is consistent with the initial discharge curve of pure graphene (inset in Fig. 3(a)). In addition, the peak between 1.5 to 0.5 V is divided into two peaks in the first cathodic process rather than in the second cathodic process, which is correlated well with the TEM analysis that the size of Cu2O is decreased in Cu2O/rGO composites [10]. Moreover, the peaks around 2.5 V are stronger and sharper, indicating that the conversion reaction between Cu2O and Li+ occurs more easily. Furthermore, the cyclic voltammogram curve of the second cycle overlaps well with that of the third cycle, suggesting that the cycling performance of Cu2O/rGO is enhanced.
For the sake of studying the function of graphene further, EIS measurements were performed. Nyquist plots of Cu2O and Cu2O/rGO electrode at open circuit voltage are presented in Fig. 4(c). Generally, it is believed that the semicircle in the medium-frequency region is assigned to the charge-transfer resistance (Rct), and the line in the lower frequency region corresponds to the Warburg impedance. It can be seen apparently that the semicircle in the medium frequency of Cu2O/rGO is smaller than that of Cu2O. According to the fitting results based on the experimental data using equivalent circuit (inset in Fig. 4(c)), the Rct is decreased from 128.01 to 84.07 Ω. The result is consistent with the electrochemical data and analysis from Fig. 3(d).
4 Conclusions
1) A facile way to synthesize Cu2O/rGO composites in aqueous solution without any surfactant instead of the commonly hydrothermal process to prepare metal oxide/rGO is developed.
2) The as-synthesized Cu2O/rGO composites are hierarchical in microscopic structure. The Cu2O particles cross-link well with rGO and show better cycling performance and rate capability than bare Cu2O when they are employed as anode materials for lithium ion batteries.
3) The existing of rGO can buffer the volume expansion and concentration as a flexible constraint and improve the conductivity of Cu2O/rGO composites.
References
[1] POIZOT P, LARUELLE S, GRUGEON S, DUPONT L, TARASCON J M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries [J]. Nature, 2000, 407(6803): 496-499.
[2] ZOU Yu-qin, JIN Kan, WANG Yong. Fe2O3-graphene rice-on-sheet nanocomposite for high and fast lithium ion storage [J]. The Journal of Physical Chemistry C, 2011, 115(42): 20747-20753.
[3] WANG Hai-liang, CUI Li-feng, YANG Yuan, CASALONGUE H S, ROBINSON J T, LIANG Yong-ye, CUI Yi, DAI Hong-jie. Mn3O4-graphene hybrid as a high-capacity anode material for lithium ion batteries [J]. Journal of the American Chemical Society, 2010, 132(40): 13978-13980.
[4] CHEN Han, FENG Fan, HU Zhong-liang, LIU Fu-sheng, GONG Wei-qiang, XIANG Kai-xiong. Preparation of uniform flowerlike CuO and flowerlike CuO/graphene composite and their application in lithium ion batteries [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 2523-2528.
[5] CHENG Bing-di, PENG Cheng-xin, CUI Zheng. Ultrasonic synthesis of CoO/graphene nanohybrids as high performance anode materials for lithiumion batteries [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 2517-2522.
[6] YANG You-ping, LIU Ren-sheng, HUANG Ke-long, WANG Li-ping, LIU Su-qin, ZENG Wen-wen. Preparation and electrochemical performance of nanosized Co3O4 via hydrothermal method [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(6): 1334-1338.
[7] GUO Hua-jun, SUN Qian-ming, LI Xin-hai, WANG Zhi-xing, PENG Wen-jie. Synthesis and electrochemical performance of Co3O4/C composite anode for lithium ion batteries [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(2): 372-376.
[8] MA Ming-you, HE Ze-qiang, XIAO Zhuo-bing, HUANG Ke-long, XIONG Li-zhi, WU Xian-ming. Synthesis and electrochemical properties of SnO2-CuO nanocomposite powders [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(4): 791-794.
[9] GRUGEON S, LARUELLE S, HERRERA-URBINA R, DUPONT L, POIZOT P, TARASCON J M. Particle size effects on the electrochemical performance of copper oxides toward lithium [J]. Journal of the Electrochemical Society, 2001, 148(4): 285-292.
[10] FU Li-jin, GAO Jian, ZHANG Tian, CAO Qian, YANG Li-cheng, WU Yu-ping, HOLZE R, WU Hao-qing. Preparation of Cu2O particles with different morphologies and their application in lithium ion batteries [J]. Journal of Power Sources, 2007, 174(2): 1197-1200.
[11] LIU Wei-jun, CHEN Gui-hua, HE Guang-hong, ZHANG Wei. Synthesis of starfish-like Cu2O nanocrystals through gamma- irradiation and their application in lithium-ion batteries [J]. Journal of Nanoparticle Research, 2011, 13(7): 2705-2713.
[12] KANG Wen-pei, LIU Feng-lin, SU Yu-lan, WANG Du-jin, SHEN Qiang. The catanionic surfactant-assisted syntheses of 26-faceted and hexapod-shaped Cu2O and their electrochemical performances [J]. Cryst Eng Comm, 2011, 13(12): 4174-4180.
[13] GEIM A K. Graphene: Status and prospects [J]. Science, 2009, 324(5934): 1530-1534.
[14] LIANG Ming-hui, ZHI Lin-jie. Graphene-based electrode materials for rechargeable lithium batteries [J]. Journal of Materials Chemistry, 2009, 19(33): 5871-5878.
[15] XU Chao, WANG Xin, YANG Li-chun, WU Yu-ping. Fabrication of a graphene–cuprous oxide composite [J]. Journal of Solid State Chemistry, 2009, 182(9): 2486-2490.
[16] ZHANG Yu, WANG Xiao, ZENG Liang, SONG Shu-yan, LIU Da-peng. Green and controlled synthesis of Cu2O-graphene hierarchical nanohybrids as high-performance anode materials for lithium-ion batteries via an ultrasound assisted approach [J]. Dalton Transactions, 2012, 41(15): 4316-4319.
[17] LI Bao-jun, CAO Hua-qiang, YIN Gui, LU Yue-xiang, YIN Jie-fu. Cu2O@reduced graphene oxide composite for removal of contaminants from water and supercapacitors [J]. Journal of Materials Chemistry, 2011, 21(29): 10645-10648.
[18] TRAN P D, BATABYAL S K, PRAMANA S S, BARBER J, WONG L H, LOOS C J. A cuprous oxide-reduced graphene oxide (Cu2O-rGO) composite photocatalyst for hydrogen generation: employing rGO as an electron acceptor to enhance the photocatalytic activity and stability of Cu2O [J]. Nanoscale, 2012, 4(13): 3875-3878.
[19] DENG S Z, TJOA V, FAN H M, TAN H R, SAYLE D C, OLIVO M, MHAISALKAR S, WEI J, SOW C H. Reduced graphene oxide conjugated Cu2O nanowire mesocrystals for high-performance NO2 gas sensor [J]. Journal of the American Chemical Society, 2012, 134(10): 4905-4917.
[20] LI D, MULLER M B, GILJE S, KANER R B, WALLACE G G. Processable aqueous dispersions of graphene nanosheets [J]. Nature Nanotechnology, 2008, 3(2): 101-105.
[21] HUMMERS W S, OFFEMAN R E. Preparation of graphitic oxide [J]. Journal of the American Chemical Society, 1958, 80(6): 1339-1339.
[22] XU Hao-lan, WANG Wen-zhong, ZHU Wei. A facile strategy to porous materials: Coordination-assisted heterogeneous dissolution route to the spherical Cu2O single crystallites with hierarchical pores [J]. Microporous and Mesoporous Materials, 2006, 95(1-3): 321-328.
[23] DU Zhi-feng, YIN Xiao-ming, ZHANG Ming, HAO Quan-yi, WANG Yan-guo, WANG Tai-hong. In situ synthesis of SnO2/graphene nanocomposite and their application as anode material for lithium ion battery [J]. Materials Letters, 2010, 64(19): 2076-2079.
[24] WU Zhong-shuai, REN Wen-cai, WEN Lei, GAO Li-bo, ZHAO Jin-ping, CHEN Zong-ping, ZHOU Guang-min, LI Feng, CHENG Hui-ming. Graphene anchored with Co3O4 nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance [J]. Acs Nano, 2010, 4(6): 3187-3194.
锂离子电池用氧化亚铜/石墨烯负极材料的制备
颜果春,李新海,王志兴,郭华军,张 倩,彭文杰
中南大学 冶金与环境学院,长沙 410083
摘 要:在不添加表面活性剂的水溶液体系中,采用水合肼作为还原剂制备得到具有八面体形貌的氧化亚铜/石墨烯复合材料。透射电镜分析表明:氧化亚铜颗粒与石墨烯在复合物中呈多层次分布,而且氧化亚铜一次颗粒很好地嵌入在石墨烯层间。相比于纯氧化亚铜,氧化亚铜/石墨烯复合材料作为锂离子电池负极材料的电化学性能得到了显著的改善。在100 mA/g的电流密度下循环50次后,氧化亚铜/石墨烯复合物的可逆比容量高达348.4 mA×h/g,同时,在不同倍率下(50, 100, 200, 400, 800 mA/g)循环60次后,其可恢复容量仍达305.8 mA×h/g。
关键词:氧化亚铜;还原氧化石墨烯;负极材料
(Edited by Hua YANG)
Foundation item: Project (2014CB643406) supported by the National Basic Research Program of China; Project (2011FJ1005) supported by Major Special Project of Science and Technology of Hunan Province, China
Corresponding author: Xin-hai LI; Tel/Fax: +86-731-88836633; E-mail: xinhai-li@csu.edu.cn
DOI: 10.1016/S1003-6326(13)62918-0
Abstract: A facile way was used to synthesize Cu2O/reduced graphene oxide (rGO) composites with octahedron-like morphology in aqueous solution without any surfactant. TEM images of the obtained Cu2O/rGOs reveal that the Cu2O particles and rGO distribute hierarchically and the primary Cu2O particles are encapsulated well in the graphene nanosheets. The electrochemical performance of Cu2O/rGOs is enhanced compared with bare Cu2O when they are employed as anode materials for lithium ion batteries. The Cu2O/rGO composites maintain a reversible capacity of 348.4 mA×h/g after 50 cycles at a current density of 100 mA/g. In addition, the composites retain 305.8 mA×h/g after 60 cycles at various current densities of 50, 100, 200, 400 and 800 mA/g.


