DOI: 10.11817/j.ysxb.1004.0609.2020-35908
脉冲电场辅助下Zn-55%Al-1.6%Si热镀层表面三价铬化学转化膜的构建
潘 杰,唐 晓,李 焰
(中国石油大学(华东) 材料科学与工程学院,青岛 266580)
摘 要:
采用化学转化法在Zn-55%Al-1.6%Si热镀层表面构建了三价铬转化膜,通过3D形貌仪、扫描电镜(SEM)、X射线光电子能谱(XPS)、拉曼光谱和接触角仪等表面分析手段,以及中性盐雾试验和电化学测试方法表征了转化膜的结构、组成和性能。利用脉冲电位法对转化膜的结构进行调控,研究了脉冲电场调控作用对转化膜结构和性能的影响。结果表明:构建的三价铬转化膜表面具有多尺度的微观形貌特征,微米级的两相结构上分布着纳米级颗粒和微孔。加载阴极脉冲方波可加速转化膜的生成,提高膜中氢氧化物的含量和膜的疏水性,还可减少膜表面的微裂纹以及Cr(Ⅵ)的含量;当脉冲方波电位φP=-100.0 mV时,构建的转化膜具有最佳的耐蚀性,能够提高对镀层表面的临时保护作用。外加脉冲电场对转化膜的结构和性能具有积极的调控作用。
关键词:
文章编号:1004-0609(2020)-11-2634-14 中图分类号:TG174.4 文献标志码:A
传统的六价铬Cr(Ⅵ)化学转化处理(Hexavalent chromium conversion passivation,CCP)由于环保法规的禁令正在逐渐退出市场,三价铬Cr(Ⅲ)甚至是无铬化学转化膜的构建与调控已成为目前环境友好型化学转化处理的主要研究热点[1-2]。其中,Cr(Ⅲ)转化膜处理(Trivalent chromium conversion passivation,TCP)在碳钢[3]、锌合金[4-6]、镀锌层[7-8]、铝合金[9-11]以及锌-铝-镁合金[12]等材料的表面均已有成功的尝试,良好的耐蚀性和成膜性均证明TCP有望成为CCP的替代方案之一。此外,TCP转化膜处理还有助于提高碳钢[3, 13]、锌合金[6]和铝合金[14]表面与环氧或聚酯底漆等有机涂层之间的结合力。为了进一步改善转化膜的成膜质量,相关研究还在铝合金[15]、镁合金[16]和钛合金[17-18]的化学转化处理过程中引入电场辅助(Electro- assisted),实现了对转化膜性能的积极调控,但是所采取的主要是恒定的电流场或施加恒定的电位。
热浸镀Zn-55%Al-1.6%Si合金镀层(以下简称Zn55Al镀层)成功地结合了锌镀层的电化学保护作用与铝镀层的阻挡层保护作用,具有优异的耐腐蚀性能,广泛应用于家电、建筑和交通等诸多领域,起到保护钢材基体的作用[19-21]。然而在更为苛刻的腐蚀环境中,热浸镀层的防护性能会逐渐衰退[22]。为进一步提高热浸镀钢板在土壤、海水、水泥等特殊环境里的耐久性,需要对其表面进行化学转化处理,然后涂装有机涂层[23]。考虑到Zn55Al热镀层表面的微观结构和组成与镀锌层、锌合金或铝合金相比具有显著差异[24-25],探索其表面Cr(Ⅲ)化学转化膜的构建及电场辅助调控具有较高的研究价值。
本文在之前的研究基础上构建了TCP转化膜[26],以脉冲电位法(Pulse potential method,PPM)[27]为辅助手段对Zn55Al镀层表面转化膜的结构和性能进行了调控,通过多种表征手段探讨了方波电位φP的影响。该方法所采用的脉冲方波电位在对热镀层表面Cr(Ⅲ)化学转化膜进行积极调控的同时,与施加恒定的电流场或阴极电位相比,降低了外电场辅助制备Cr(Ⅲ)化学转化膜过程中的能耗,提高了效率,研究结果对于生产有一定的指导意义。
1 实验
1.1 试样制备
实验采用厚度为1.2 mm的市售热浸镀Zn-55%Al-1.6%Si钢板。试样尺寸为50 mm×50 mm,镀层厚度为25 μm。试样经去离子水冲洗、25 ℃丙酮超声清洗20 min、常温无水乙醇超声清洗10 min后,冷风吹干放入干燥器备用。
化学转化处理采用Bonderite M-NT 5928商业溶液(含有质量分数为1%~10%的三价铬盐和质量分数为0.1%~1%的锆酸盐),经高纯水稀释1倍,pH为2.6[26]。化学转化处理温度为40 ℃,总的成膜时间为180 s,转化结束后用去离子水清洗试样并在室温下干燥保存。
M-NT 5928商业溶液(含有质量分数为1%~10%的三价铬盐和质量分数为0.1%~1%的锆酸盐),经高纯水稀释1倍,pH为2.6[26]。化学转化处理温度为40 ℃,总的成膜时间为180 s,转化结束后用去离子水清洗试样并在室温下干燥保存。
PPM辅助成膜采用矩形方波,电位值φP分别设置为-50.0、-100.0和-200 mV(vs φocp),对应的平均电流密度大小分别为-1.5、-3.0和-8.0 mA/cm2,频率f为5 Hz,通断比为0.2 s:0.2 s。试样在自然电位下成膜20 s后开始施加脉冲电位,总的成膜时间保持不变。
1.2 表面分析
通过Zeta-20A 3D型表面形貌仪测量试样的表面形貌并计算线粗糙度Ra(轮廓算术平均偏差)和Rz(轮廓最大高度差),物镜放大倍数为100倍,宏观粗糙度线扫距离L为200 μm,激光束扫描的步长为0.027 μm,Z轴景深为4~5 μm。通过JSM-7200F型扫描电镜(SEM)获取试样表面的微观形貌信息,加速电压为15 kV。采用XG-CAMC型接触角测量仪在室温下测量去离子水溶液在试样表面的润湿性,所有接触角的测量在同一试样的其他位置重复5次。通过ESCALAB 250Xi型X射线光电子能谱仪获取试样表面转化膜的组成信息,X射线源为Al Kα微聚焦单色器,光斑直径400 μm,探测深度2 nm,Cr 2p精扫图谱采用C 1s峰以284.6 eV进行校正。采用Horiba Lab-RAM HR Evolution型拉曼光谱仪获得试样表面200~1200 cm-1范围内的拉曼光谱,测试能量小于0.5 mW,通过标准硅片在520 cm-1处拉曼位移进行校正,每个试样分别测试3个点位,每个点位累积积分10次避免杂散光噪声的影响。
1.3 腐蚀和电化学测试
中性盐雾试验按照标准ASTM B117—2002进行。溶液采用质量分数为5 %的NaCl水溶液,盐雾箱温度控制为35 ℃,腐蚀周期为8 d,喷雾量为50 mL/(cm2·d),拍照记录前去除试样表面的腐蚀产物并冲洗烘干。
电化学测试采用Autolab PGSTAT302F型电化学工作站进行。工作电极为经化学转化处理的试样,其工作面积为1.0 cm2,参比电极为Ag/AgCl(饱和氯化钾)参比电极,辅助电极为大面积Pt片。实验在室温下进行,腐蚀电解质为0.5 mol/L NaCl水溶液,未做除氧处理。电化学阻抗谱测量采用幅值为10 mV的交流正弦波信号,测试频率为105~10-2 Hz,每个倍频范围内测试10个点,数据通过软件Nova 2.1进行拟合。弱极化曲线测量的电位扫描范围为-60~60 mV(vs φocp),扫描速率为0.166 mV/s,通过三参数方程进行极化曲线拟合。
2 实验结果
2.1 TCP转化膜的宏观形貌
图1所示为Zn55Al镀层表面的二维形貌图。如图1(a)所示,脱脂后的镀层表面存在着两相结构,交替分布着微米级的富铝枝晶区和富锌枝晶间区[21]。如图1(b)所示,普通化学转化法构建的TCP转化膜呈墨绿色,镀层表面形貌由平整光滑变为粗糙。当引入脉冲电场辅助成膜且脉冲方波电位φP=200.0 mV时,由图1(c)可见,转化膜在枝晶和枝晶间区的形貌差异较大;而当脉冲方波电位φP=-200.0 mV时(见图1(d)),所构建的转化膜在枝晶区和枝晶间区的完整性和均一性都优于图1(b)和(c)中的转化膜。
图2所示为Zn55Al镀层表面局部轮廓曲线和相应的微观粗糙度。由图2可知,当φP=-200.0 mV时,Zn55Al表面枝晶区的微观粗糙度增加了2~3倍,且轮廓最大高度差Rz降低了30.9%。表1所示为镀层试样表面宏观线粗糙度Ra测量结果。由表1可见,与未处理试样相比,构建转化膜后表面的宏观线粗糙度降低了19.0%。经阴极脉冲电方波电位调控后,转化膜表面宏观线粗糙度降低了62.6%;而阳极脉冲方波电位的辅助仅使试样表面宏观线粗糙度降低了11.6%。由此可见,引入脉冲电场辅助成膜后,Zn55Al镀层表面转化膜在相间的宏观不平整度降低,而相内的微观粗糙度显著提高。
2.2 TCP转化膜的微观形貌
图3所示为图1中对应的Zn55Al镀层表面微米级两相结构的微观形貌。在成膜之前,试样表面(见图3(a)和(b))的枝晶区较为光滑、平整,富锌枝晶间区分布颗粒状析出相,存在少量直径约为2 μm的深孔。当试样表面构建了TCP转化膜后,如图3(c)所示,枝晶间区部分颗粒析出相消失,呈现出纳米级多孔结构;枝晶区呈织构状,密布着较深的细小微孔和少量的微裂纹(见图3(d)),表明Zn55Al镀层表面构建的转化膜具有多尺度微结构特征,即微米级的两相结构上分布着纳米级颗粒和微孔。当脉冲电位φP=-50.0 mV时,如图3(e)所示,枝晶间区的微孔数量和尺寸减小;而枝晶区的微裂纹尺寸明显增大,表明转化膜的厚度有所增大(见图3(f))。当脉冲电位增大至φP=-100.0 mV时,如图3(g)所示,枝晶间区由于有多孔状的转化膜析出长大而变粗糙,枝晶和枝晶间区的宏观不平整度降低;而枝晶区的微裂纹减少,密布着直径为~100 nm的球状颗粒(见图3(h)),表明脉冲电场的调控使转化膜表面的微结构发生了改变。
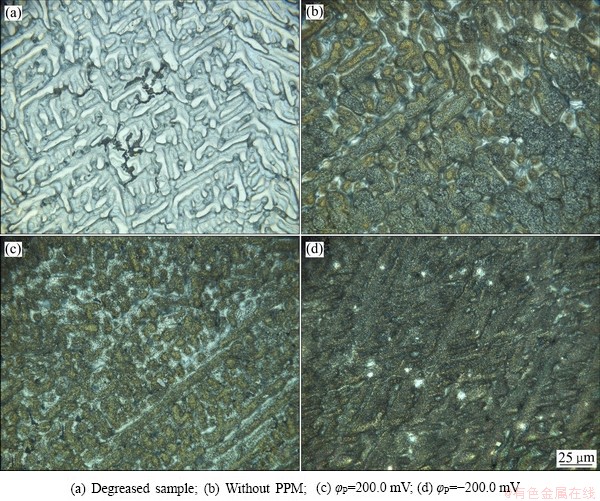
图1 Zn55Al镀层表面TCP转化膜的二维形貌图
Fig. 1 2D morphologies of TCP conversion coatings on surface of Zn55Al samples
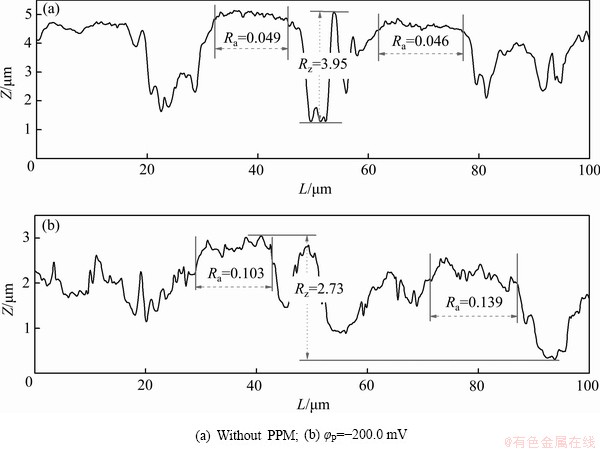
图2 Zn55Al镀层表面TCP转化膜的局部轮廓线和微观粗糙度
Fig. 2 Local contour and micro roughness Ra of TCP conversion coatings on surface of Zn55Al samples
表1 不同条件下Zn55Al镀层表面宏观线粗糙度Ra
Table 1 Macro line roughness Ra on surface of Zn55Al samples under different conditions
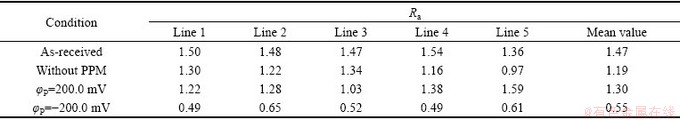
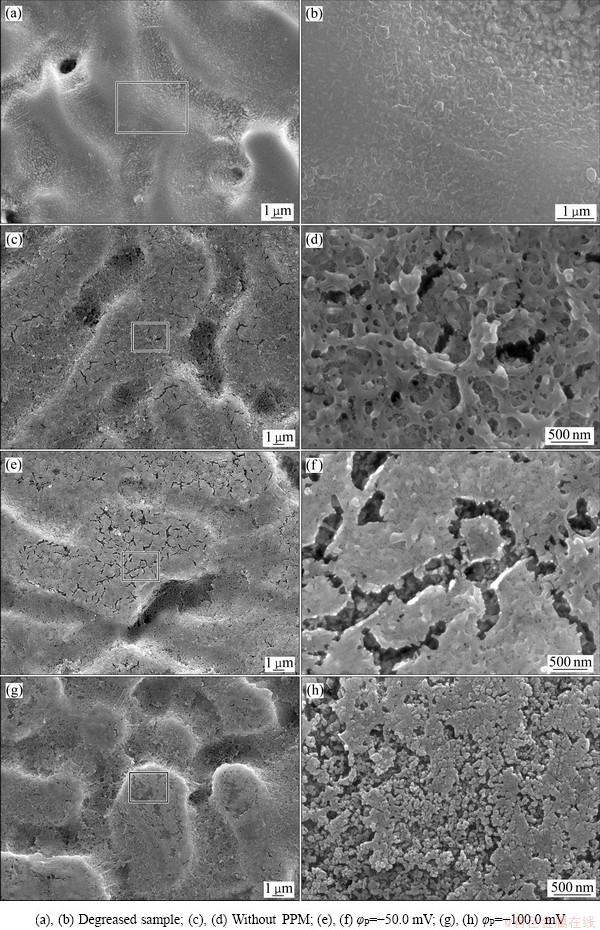
图3 Zn55Al镀层表面TCP转化膜微观形貌
Fig. 3 SEM morphologies of TCP conversion coatings on surface of Zn55Al samples
研究表明[28-29],锌铝合金表面的化学转化膜对微裂纹十分敏感,微裂纹的出现在一定程度上会降低转化膜的耐蚀性,尤其是在高氯的溶液或微液滴腐蚀环境中;微裂纹的出现一方面是由于生成的转化膜厚度增大,另一方面则是来源于扫描电镜的真空环境使得转化膜脱水而产生开裂。脉冲辅助电场可以在一定程度上消除转化膜内部的应力集中,减少转化膜表面的微裂纹[30]。
2.3 TCP转化膜的组成
图4(a)、(c)、(e)所示为Zn55Al镀层表面TCP转化膜的XPS通谱,其中C和O的含量较高,其他成膜元素包括Al、Zn、Cr、Zr和F等。表2所示为经去除C污染校正后的转化膜元素构成。可见,经脉冲电场调控后,膜中的Cr元素含量明显增加。阴极脉冲电位的施加促进了成膜反应的阴极过程,且加快了转化液中Cr3+、Zr4+与OH-的结合;增加了转化膜中Cr、Zr元素的含量[28, 31]。
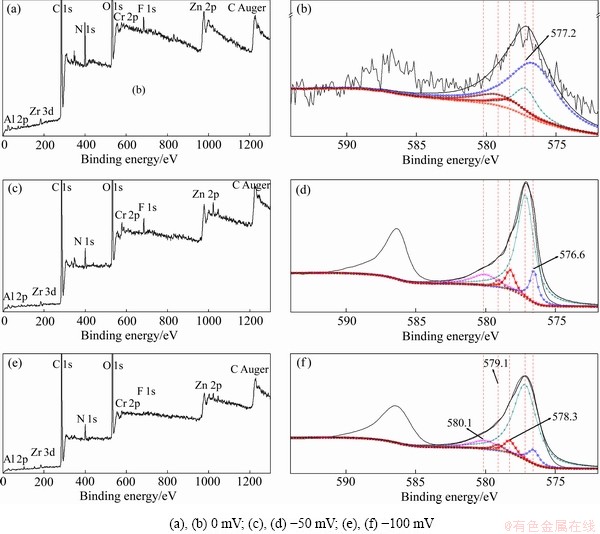
图4 不同脉冲电位φP下Zn55Al镀层表面TCP转化膜的XPS通谱和Cr 2p3/2 拟合结果
Fig. 4 High resolution Cr 2p3/2 spectra and XPS full spectra of TCP conversion coatings on surface of Zn55Al samples with different φP values
表2 不同脉冲电位φP下TCP转化膜中主要元素的摩尔分数
Table 2 Mole fractions of main elements in TCP conversion coatings with different φP values
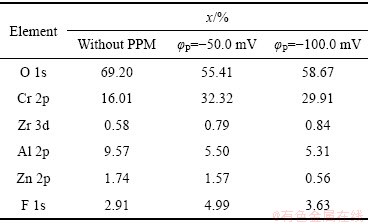
表3 不同脉冲电位φP下TCP转化膜Cr 2p3/2谱中各物质峰面积占比
Table 3 Peak area proportion of Cr 2p3/2components of TCP conversion coatings with different φP values
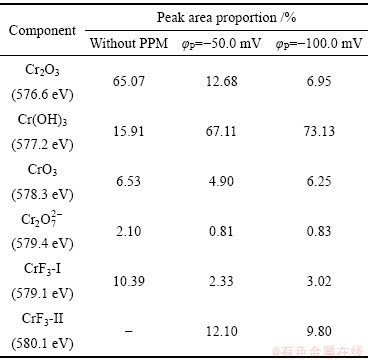
图4(b)、(d)、(f)所示为Zn55Al镀层表面TCP转化膜的Cr 2p3/2 XPS高分辨谱及拟合结果,其中576.6 eV表示Cr2O3[32],577.2 eV表示Cr(OH)3[33],578.3和579.4 eV分别代表Cr(Ⅵ)物质CrO3和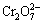 [34-35],579.1和580.1 eV表示CrF3[33, 36],表3列出了各种含Cr物质在膜中的占比。可见,新生的TCP转化膜中会出现痕量的Cr(Ⅵ)物质,但当φP分别为-50.0和-100.0 mV时,Cr(Ⅵ)的含量分别降低了33.83%和17.96%。脉冲电场的施加显著提高了Cr 2p3/2能带区域的峰强,且Cr(Ⅲ)氢氧化物的含量由15.91%提高到67.11%和73.13%。此外,在转化液中F-的作用下,部分金属氢氧化物还会转变为氟化物,使得TCP转化
[34-35],579.1和580.1 eV表示CrF3[33, 36],表3列出了各种含Cr物质在膜中的占比。可见,新生的TCP转化膜中会出现痕量的Cr(Ⅵ)物质,但当φP分别为-50.0和-100.0 mV时,Cr(Ⅵ)的含量分别降低了33.83%和17.96%。脉冲电场的施加显著提高了Cr 2p3/2能带区域的峰强,且Cr(Ⅲ)氢氧化物的含量由15.91%提高到67.11%和73.13%。此外,在转化液中F-的作用下,部分金属氢氧化物还会转变为氟化物,使得TCP转化
膜中氟化物的含量也相应地增加:
MZn,Al,Cr(OH)n+xF-→ +nOH- (1)
+nOH- (1)
而转化液中的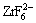 还会发生水解反应生成稳定致密的ZrO2氧化物分布于转化膜之中:
还会发生水解反应生成稳定致密的ZrO2氧化物分布于转化膜之中:
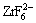 +4OH-→ZrO2·2H2O+6F- (2)
+4OH-→ZrO2·2H2O+6F- (2)
2.4 TCP转化膜的耐蚀性
2.4.1 弱极化曲线
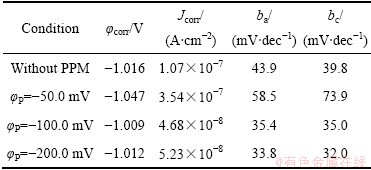
图5所示为Zn55Al镀层在0.5 mol/L NaCl溶液中所测得的弱极化曲线,利用三参数公式[37]对其进行拟合得到的动力学参数如表4所示。与未经外场辅助成膜的试样相比,当φP为-50和-100 mV时,表面构建了转化膜的镀层试样在盐水中的腐蚀电流密度降低了近一个数量级,且当脉冲电位φP=-100.0 mV时得到的腐蚀电流最小。可见,施加适当的阴极脉冲方波电位可以进一步改善转化膜的耐蚀性。
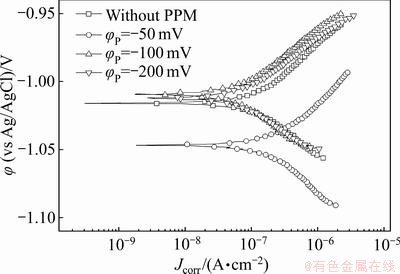
图5 不同脉冲电位φP下Zn55Al镀层表面TCP转化膜在0.5 mol/L NaCl溶液中的弱极化曲线
Fig. 5 Weak polarization curves of TCP conversion coatings on surface of Zn55Al samples with different φP in 0.5 mol/L NaCl solution
表4 Zn55Al镀层表面TCP转化膜在0.5 mol/L NaCl溶液中弱极化曲线各动力学参数拟合结果
Table 4 Fitting results of kinetic parameters for weak polarization curve measurements of TCP conversion coatings on surface of Zn55Al samples in 0.5 mol/L NaCl solution
2.4.2 电化学阻抗
图6所示为Zn55Al镀层在0.5 mol/L NaCl溶液中的Nyquist图和Bode图,采用图7所示的两时间常数的等效电路进行拟合。其中,Rs代表溶液电阻;Cdl代表多孔隙转化膜底部电化学步骤的双电层电容;Rct表示电荷转移电阻;Rpore,sol和Cf分别表示孔隙膜电阻和电容。等效电路中两个时间常数的容抗弧并联能有效反映镀层表面及其氧化膜在NaCl溶液中的电化学响应[38-40]。由图6可见,构建TCP转化膜可以提高试样表面的耐蚀性,施加阴极脉冲方波电位可以进一步改善转化膜的耐蚀性,且当脉冲电位φP=-100.0 mV时,Nyquist图中实部和Bode图中|Z|的数值最大,表明适当的脉冲电场辅助成膜可显著提升Zn55Al镀层表面构建转化膜的耐蚀性。这与弱极化曲线测量的结果是一致的。低频区出现的由浓差极化导致的Warburg阻抗,表明转化膜成膜后Zn55Al在盐水中的腐蚀受到有限的氧扩散控制[41-42]。
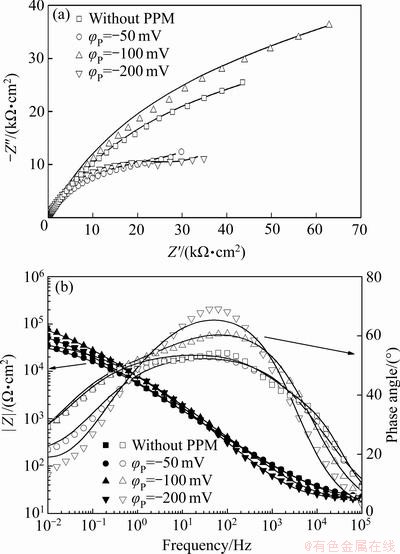
图6 不同脉冲电位φP下Zn55Al镀层表面转化膜在0.5 mol/L NaCl溶液中的Nyquist图和Bode图
Fig. 6 Nyquist diagram (a) and Bode plots (b) of TCP conversion coatings on surface of Zn55Al samples with different φP values in 0.5 mol/L NaCl solution
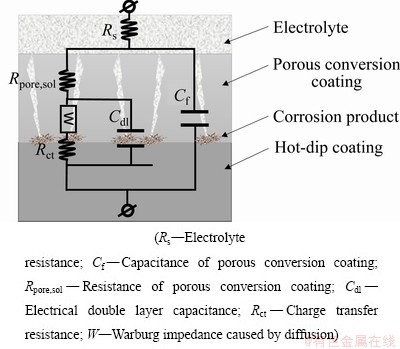
图7 EIS等效电路图
Fig. 7 Equivalent circuits for fitting EIS data
表5所示为拟合得到的电化学阻抗测试结果[43]。可见,当脉冲电位φP=-100.0 mV时,试样的Rpore,sol+Rct值大于其他条件下成膜试样的Rpore,sol+Rct值,其中电荷转移电阻Rct (1.15×105 Ω·cm2)增大了近1个数量级。Rct值越大,表明电荷在界面和溶液中的传输困难度越大,转化膜的阻隔作用越好。同时,当φP=-100.0 mV时拟合得到的Cf和Cdl值最小,表明转化膜表面更均匀,转化膜的致密性与耐蚀性较好[44-47]。
2.4.3 中性盐雾腐蚀
图8所示为Zn55Al镀层试样在中性盐雾实验后的腐蚀形貌。未经脉冲电场调控的转化膜表面在4 d开始出现明显的局部腐蚀,形成白色的腐蚀产物[48] (见图8(a′)),8 d时试样腐蚀程度加剧(见图8(a″));而脉冲电场调控后的转化膜与此相比则表现出了良好的耐蚀性。与电化学测试的结果一致,当脉冲方波电位φP=-100.0 mV时,转化膜的耐蚀性最佳。
表5 不同脉冲电位φP下TCP转化膜在0.5 mol/L NaCl溶液中的EIS拟合结果
Table 5 EIS fitting results of TCP conversion coatings with different φP values in 0.5 mol/L NaCl solution
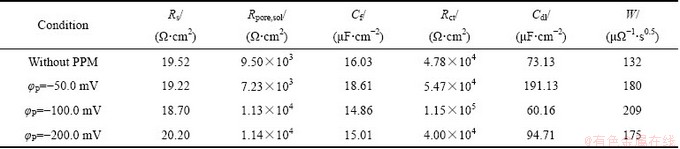
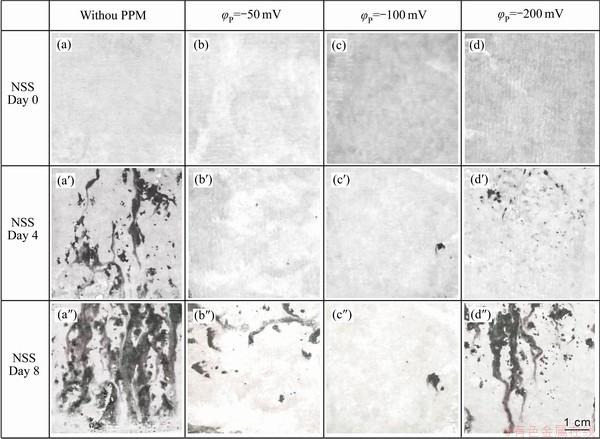
图8 不同成膜条件下TCP转化膜在中性盐雾试验中腐蚀8 d后的表面形貌
Fig. 8 Surface morphologies of TCP conversion coatings under different film-forming conditions after 8 d corrosion in neutral salt spray test

图9 不同脉冲电位φP和成膜时间下TCP转化膜在中性盐雾试验中腐蚀8 d后的表面形貌
Fig. 9 Surface morphologies of TCP conversion coatings under different φP values and film-forming time after 8 d corrosion in neutral salt spray test
研究[26, 28]表明,成膜时间对Zn55Al镀层表面TCP转化膜的耐蚀性有着比较显著的影响,因此图9对比了未经外场辅助时成膜300 s和在φP=-100.0 mV时成膜180 s的试样在中性盐雾中腐蚀8 d后的形貌。由图9可见,脉冲电场调控后的试样与更长时间下构建的转化膜具有相当的耐蚀性,显然,这对提高Zn55Al镀层化学转化处理的作业效率和减少能源的消耗是具有积极意义的。
3 分析讨论
3.1 TCP转化膜的构建过程和脉冲电场的调控作用
图10所示为Zn55Al镀层表面TCP转化膜的构建过程中开路电位随时间的变化情况。在Zn55Al镀层表面TCP转化膜的构建初期,Zn55Al镀层表面的富锌枝晶间区为主阳极区,而富铝枝晶区为主阴极区。随着阳极反应(3)和阴极反应(4)和(5)的进行[28-29],开路电位快速负移,并在20 s时逐渐稳定在-0.916 V左右。镀层表面的Zn和Al依次被酸性转化液活化为Zn2+和Al3+进入转化液中,而主阴极区附近则富集了较多的OH-。
阳极:
MZn,Al→MZn,Aln++ne (3)
阴极:
2H++2e→H2 (4)
O2+2H2O+4e→4OH- (5)
在成膜过程中,主阳极区表面的阳极反应占优,且Zn组元溶解速度大于Al组元,因此在枝晶间区形成了如图3(c)所示的微孔结构;而在主阴极区表面主要发生阴极反应,OH-含量较高,溶液中的Zn2+、Al3+和Cr3+等金属阳离子与OH-结合,率先在枝晶区形成了氢氧化物沉淀膜,由于转化膜厚度较高,脱水后易出现较为粗大的微裂纹:
MZn,Al,Crn++nOH-→MZn,Al,Cr(OH)n (6)
当混合电位趋于稳定后,如图10所示,利用脉冲方波电位法对转化膜的构建过程进行调控,可使转化膜具备更为明显的多尺度特征(见图3)。加载的阴极方波电位一定程度上抑制了镀层表面尤其是主阳极区内Zn和Al的溶解,使枝晶间区的微孔尺寸减小;而枝晶区表面的阴极过程则得以加速,OH-含量进一步增大,经由异相形核形成了更多的纳米级球状颗粒,减少了表面由于膜厚较高而引起的微裂纹(见图3(g)和(h))[29, 49],并且阴极电位也有助于部分Cr6+被还原为Cr3+,这些都会加速TCP的成膜过程[50]。此外,施加阴极方波电位后,大量生成的OH-向枝晶间区迁移,使转化膜在枝晶间区加速析出,降低了枝晶与枝晶间的宏观粗糙度。
由表1和图2可知,转化膜表面的宏观粗糙度大幅降低而微观粗糙度显著增大,这有助于提高涂层在Zn55Al镀层表面的附着力。宏观粗糙度的减小有利于涂层在试样表面的铺展,而粗糙的表面微观孔隙则有助于提高涂层在试样表面的机械嵌合力,起到“锚合”的效果[6, 13-14]。此外,经过脉冲电场调控后的转化膜所具备的这种多尺度微结构特征还有助于提高镀层表面的疏水性[51]。图11所示为Zn55Al镀层表面微液滴接触角测试结果。由图11可见,未经处理的Zn55Al热镀层因其表面具有一层耐指纹涂层因而具有一定的疏水性,接触角约为106.3°,脱脂处理后表面接触角变为98.0°;常规转化方法构建的转化膜接触角仅为84.5°,而Zn55Al镀层表面的转化膜在经脉冲电场调控后,接触角提高到109.3°。这表明脉冲电场的调控作用对疏水性转化膜的构建也有一定的潜在研究价值。
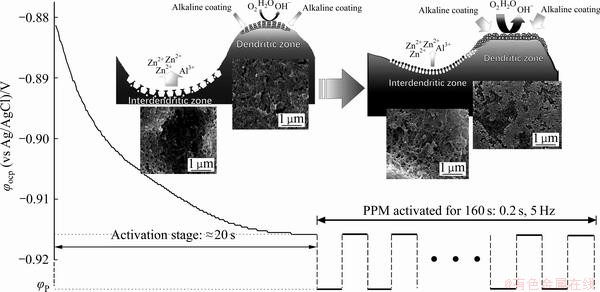
图10 脉冲电场调控下Zn55Al镀层表面TCP转化膜的成膜机理示意图
Fig. 10 Schematic diagram of film formation mechanism of TCP conversion coatings on surface of Zn55Al samples after applying PPM

图11 不同成膜条件下Zn55Al镀层表面2 μL微液滴接触角测试结果
Fig. 11 Contact angle measurements of 2 μL water droplet on surface of Zn55Al samples under different film-forming conditions
由表2所示转化膜中的元素含量可知,在脉冲电场的调控作用下,Zn55Al镀层表面构建的TCP转化膜中Cr和Zr的含量均有所增加。考虑到Cr和Zr的氧化物和氢氧化物在转化膜中起着隔绝腐蚀介质对基体侵蚀的主要作用[29],因此,经脉冲电场辅助成膜且当φP=-100 mV时,试样表现出如图5、6和8所示的良好耐蚀性。此外,由表3给出的转化膜中Cr的分布情况看,其氢氧化物的含量显著提高,这对提高转化膜与基底和底漆的结合力也是非常有利的[6]。
3.2 脉冲电场对TCP转化膜中Cr(Ⅵ)物质的影响
新生的Cr(Ⅲ)-锆转化膜中会出现痕量的Cr(Ⅵ),这与成膜过程中H2O2的生成有着直接关系。研究表明,转化过程中的阴极反应容易生成少量的H2O2,而H2O2易于扩散到新生转化膜附近,将不溶性Cr(OH)3氧化成可溶性Cr(Ⅵ)物质(如: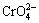 ),并以溶液中的金属阳离子结合为铬酸盐沉淀进入Cr(Ⅲ)化学转化膜[52]。
),并以溶液中的金属阳离子结合为铬酸盐沉淀进入Cr(Ⅲ)化学转化膜[52]。
生成H2O2:
O2+2H++2e→H2O2 (7)
生成Cr(Ⅵ)物质:
2Cr(OH)3+3H2O2+4OH-→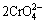 +8H2O (8)
+8H2O (8)
但是,2e参与的H2O2的生成需要较高的能量和纳米级的双金属电催化剂[53],因此转化膜中Cr(Ⅵ)出现的机理仍待探索。尽管如此,由于Cr(Ⅵ)→Cr(Ⅲ)的还原电位(1.330 V vs SHE)高于O2(0) →O2(-1)的还原电位(0.695 V vs SHE),相对于反应式(7),脉冲方波电位的施加促进了Cr(Ⅵ)在阴极自发还原为Cr(Ⅲ)的反应式(9),使得生成膜层附近溶液中的Cr(Ⅵ)物质有所减少[54]:
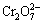 +14H++6e→2Cr3++7H2O (9)
+14H++6e→2Cr3++7H2O (9)
本文采用脉冲电场对转化膜的构建进行了调控,促进Cr(Ⅵ)向Cr(Ⅲ)还原。图12所示为Zn55Al镀层表面TCP转化膜的拉曼光谱。由图12可见,510~710 cm-1处的峰带代表Cr(Ⅲ)物质,其中556、620和710 cm-1处的峰表示含有Cr(Ⅲ)—O键的Cr2O3[55-56],而包含Cr(Ⅲ)的氢氧化物如Cr(OH)3或CrOOH等则因水合状态的不同而分布于510~556 cm-1之间[57-58]。388 cm-1和800~870 cm-1处峰位表示含有Cr(Ⅵ)—O键的Cr(Ⅵ)物质,如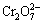 [58-59]。此外,476 cm-1处峰位表示ZrO2,268和320 cm-1处峰位表示CrF3[35, 60]。如图12所示,Cr(Ⅵ)物质的峰(388 cm-1和800~870 cm-1)有所减弱,这一结果与XPS分析结果共同表明,在阴极性脉冲电位的作用下,转化膜中Cr(Ⅵ)物质的含量降低,而Cr(Ⅲ)的氧化物和氢氧化物等主要成膜物质的生成得到加强。这有助于提升热镀层表面Cr(Ⅲ)化学转化膜的环境友好性,扩大其在金属表面预处理领域的应用前景与价值。鉴于脉冲电位法对Zn55Al镀层表面构建转化膜结构和性能呈现出积极的调控作用,且这一方法的影响因素较多,因此,对脉冲电位法中方波电位φP值、通断比和频率f等主要参数的优化正在进行中。
[58-59]。此外,476 cm-1处峰位表示ZrO2,268和320 cm-1处峰位表示CrF3[35, 60]。如图12所示,Cr(Ⅵ)物质的峰(388 cm-1和800~870 cm-1)有所减弱,这一结果与XPS分析结果共同表明,在阴极性脉冲电位的作用下,转化膜中Cr(Ⅵ)物质的含量降低,而Cr(Ⅲ)的氧化物和氢氧化物等主要成膜物质的生成得到加强。这有助于提升热镀层表面Cr(Ⅲ)化学转化膜的环境友好性,扩大其在金属表面预处理领域的应用前景与价值。鉴于脉冲电位法对Zn55Al镀层表面构建转化膜结构和性能呈现出积极的调控作用,且这一方法的影响因素较多,因此,对脉冲电位法中方波电位φP值、通断比和频率f等主要参数的优化正在进行中。
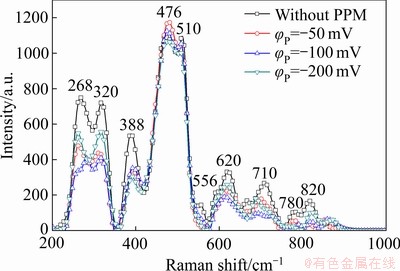
图12 不同脉冲电位φP下Zn55Al镀层表面TCP转化膜的拉曼光谱
Fig. 12 Ramen spectra of TCP conversion coatings on surface of Zn55Al samples with different φP values
4 结论
1) Zn55Al镀层表面构建的TCP转化膜具有多尺度的微结构特征,微米级的两相结构上分布着纳米级颗粒和微孔。阴极脉冲方波电位的调控显著增大了试样表面的微观粗糙度,降低了试样表面的宏观粗糙度;当脉冲方波电位φP=-100.0 mV时,转化膜表面的微裂纹较少,且转化膜表面具备一定的疏水性,接触角达到109.3°。
2) 脉冲电场降低了Zn55Al镀层表面TCP转化膜中Cr(Ⅵ)物质的含量,加速了转化膜的生成,转化膜中Cr、Zr元素和其碱性氢氧化物的含量均有所增加。当φP=-50.0 mV时,Cr(Ⅵ)物质的含量降低了33.83%,而Cr(Ⅲ)氢氧化物的含量增加了51.20%。
3) 脉冲电场提高了Zn55Al镀层表面TCP转化膜的耐蚀性。当φP=-100.0 mV时,Zn55Al镀层试样在盐水中的腐蚀电流密度最低,阻抗值最高,耐中性盐雾时长可达8 d,均优于普通化学转化构建的转化膜。
REFERENCES
[1] LI Y, WANG H, HOU B, FENG F, WEI X. Chromate passivation of hot dipped Zn25Al alloy coatings[J]. British Corrosion Journal, 2001, 36(1): 56-58.
[2] KENDIG M W, BUCHHEIT R G. Corrosion inhibition of aluminum and aluminum alloys by soluble chromates, chromate coatings, and chromate-free coatings[J]. Corrosion, 2003, 59(5): 379-400.
[3] SABABI M, TERRYN H, MOL J M C. The influence of a Zr-based conversion treatment on interfacial bonding strength and stability of epoxy coated carbon steel[J]. Progress in Organic Coatings, 2017, 105: 29-36.
[4] TAHERI P, LAHA P, TERRYN H, MOL J M C. An in situ study of zirconium-based conversion treatment on zinc surfaces[J]. Applly Surface Science, 2015, 356: 837-843.
[5] SHEU H H, LEE H B, JIAN S Y, HSU C Y, LEE C Y. Investigation on the corrosion resistance of trivalent chromium conversion passivate on electroplated Zn-Ni alloy[J]. Surface and Coatings Technology, 2016, 305: 241-248.
[6] TAHERI P, LILL K, DE WIT J H W, MOL J M C, TERRYN H. Effects of zinc surface acid-based properties on formation mechanisms and interfacial bonding properties of zirconium-based conversion layers[J]. The Journal of Physical Chemistry C, 2012, 116(15): 8426-8436.
[7] GAO Z Q, ZHANG D W, LI X G, JIANG S M, ZHANG Q F. Current status, opportunities and challenges in chemical conversion coatings for zinc[J]. Colloids and Surfaces A (Physicochemical and Engineering Aspects), 2018, 546: 221-236.
[8] LIU X H, WANG M, LI H X, WANG L B, XU Y. Electrochemical effects of pH value on the corrosion inhibition and microstructure of cerium doped trivalent chromium conversion coating on Zn[J]. Corrosion Science, 2020, 167: 1-11. doi.org/10.1016/j.corsci.2020.108538.
[9] MUNSON C A, MCFALL-BOEGEMAN S A, SWAIN G M. Cross comparison of TCP conversion coating performance on aluminum alloys during neutral salt-spray and thin-layer mist accelerated degradation testing[J]. Electrochimica Acta, 2018, 282: 171-184
[10] VIROULAUD R, SWIATOWSKA J, SEYEUX A, ZANNA S, TARDELLI J, MARCUS P. Influence of surface pretreatments on the quality of trivalent chromium process coatings on aluminum alloy[J]. Applied Surface Science, 2017, 423: 927-938.
[11] VERDALET-GUARDIOLA X, FORIC B, BONINOB J P, DULUARDB S, BLANC C. Nucleation and growth mechanisms of trivalent chromium conversion coatings on 2024-T3 aluminium alloy[J]. Corrosion Science, 2019, 155: 109-120.
[12] LOSTAK T, MALJUSCH A, KLINK B, KREBS S, KIMPEL M, FLOCK J, SCHULZ S, SCHUHMANN W. Zr-based conversion layer on Zn-Al-Mg alloy coated steel sheets: insights into the formation mechanism[J]. Electrochimica Acta, 2014, 137: 65-74.
[13] GHANBARI A, ATTAR M M. Surface free energy characterization and adhesion performance of mild steel treated based on zirconium conversion coating: A comparative study[J]. Surface and Coatings Technology, 2014, 246: 26-33.
[14] SHARIFI GOLRU S, ATTAR M M, RAMEZANZADEH B. Effects of surface treatment of aluminium alloy 1050 on the adhesion and anticorrosion properties of the epoxy coating[J]. Applly Surface Science, 2015, 345: 360-368.
[15] DONG X, ARGEKAR S, WANG P, SCHAEFER D W. In situ evolution of trivalent chromium process passive film on Al in a corrosive aqueous environment[J]. ACS Appl Mater Interfaces, 2011, 3(11): 4206-4214.
[16] BA Zhi-xin, ZHANG, Xiao-bo, WANG Zhang-zhong, FANG Xin-xian, DONG Qiang-sheng, WANG Q. Electric field assisted chemical conversion process of AZ91D magnesium alloy in HCO3-/CO32- solution[J]. Transaction of Nonferrous Metals Society of China, 2014, 24: 3818-3824.
[17] ZHAO X C, HUANG B X, MA J, CHEN H, WANG C Z, LU Y P. Study on influencing factors and mechanisms of electro-assisted chemical conversion on titanium[J]. Materials Letters, 2019, 250: 108-111.
[18] ZHAO X C, DONG S F, GE B, HUANG B X, MA J, CHEN H, HAO X H, WANG C Z. Effects of temperature and voltage on formation of electrolysis induced chemical conversion coating on titanium surface[J]. Surface and Coatings Technology, 2018, 354: 330-341.
[19] PANOSSIAN Z, MARIACA L, MORCILLO M, FLORES S, ROCHA J, PENA J J, HERRERA F, CORVO F, SANCHEZ M, RINCON O T, PRIDYBAILO G, SIMANCAS J. Steel cathodic protection afforded by zinc, aluminium and zinc/aluminium alloy coatings in the atmosphere[J]. Surface and Coatings Technology, 2005, 190(2/3): 244-248.
[20] 袁训华, 林 源, 张启富. 热镀锌铝镁镀层的切边保护性能和耐腐蚀机理[J]. 中国有色金属学报, 2015, 25(9): 2453-2463.
YUAN Xun-hua, LIN Yuan, ZHANG Qi-fu. Cut-edge protection performance and corrosion resistance mechanisms of galvanized Zn-Al-Mg alloy coating[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(9): 2453-2463.
[21] JU H, LI Y. Nicotinic acid as a nontoxic corrosion inhibitor for hot dipped Zn and Zn-Al alloy coatings on steels in diluted hydrochloric acid[J]. Corrosion Science, 2007, 49(11): 4185-4201.
[22] 范嘉雯, 程学群, 李晓刚, 肖 葵, 董超芳. 纯锌在我国热带海洋大气环境耐蚀寿命预测模型[J]. 中国有色金属学报, 2016, 26(4): 797-806.
FAN Jia-wen, CHENG Xue-qun, LI Xiao-gang, XIAO Kui, DONG Chao-fang. Corrosion prediction model of pure zinc in tropical marine atmospheric environment in China[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(4): 797-806.
[23] 章小鸽. 镀锌保护钢铁的效率和新型锌镀层的发展前景[J]. 中国腐蚀与防护学报, 2010, 30(2): 166-170.
ZHANG Xiao-ge. Efficiency of corrosion protection of steel by galvanizing and prospect for new coating development[J]. Journal of Chinese Society for Corrosion and Protection, 2010, 30(2): 166-170.
[24] 李 焰, 邢少华, 李 鑫, 魏绪钧. 热浸镀层在青岛站的海水腐蚀行为对比(Ⅲ)—飞溅区[J]. 中国有色金属学报, 2007, 17(9): 1527-1535.
LI Yan, XING Shao-hua, LI Xin, WEI Xu-jun. Seawater corrosion behavior of hot dip coatings at Qingdao test station (Ⅲ)—Splash zone[J]. The Chinese Journal of Nonferrous Metals, 2007, 17(9): 1527-1535.
[25] PERSSON D, THIERRY D, KARLSSON O. Corrosion and corrosion products of hot dipped galvanized steel during long term atmospheric exposure at different sites world-wide[J]. Corrosion Science, 2017, 126: 152-165.
[26] PAN J, TANG X, LI Y. Influence of treatment time on performance of Cr(Ⅲ)-based conversion coatings on hot dip Zn-55Al-1.6Si coated steel sheet[J]. Coatings, 2019, 9(5): 1-22.
[27] SARAVANAN G, MOHAN S. Corrosion behavior of Cr electrodeposited from Cr(Ⅵ) and Cr(Ⅲ)-baths using direct (DCD) and pulse electrodeposition (PED) techniques[J]. Corrosion Science, 2009, 51(1): 197-202.
[28] CHANG Yu-Tsern, WEN Niann-Tsyr, CHEN We-Kun, GER Ming-Der, PAN Guan-Tin, YANG Thomas C-K. The effects of immersion time on morphology and electrochemical properties of the Cr(Ⅲ)-based conversion coatings on zinc coated steel surface[J]. Corrosion Science, 2008, 50(12): 3494-3499.
[29] GUO Y, FRANKEL G S. Characterization of trivalent chromium process coating on AA2024-T3[J]. Surface and Coatings Technology, 2012, 206(19/20): 3895-3902.
[30] MARTYAK N M. Internal stresses in zinc-chromate coatings[J]. Surface and Coatings Technology, 1996, 88(1/3): 139-146.
[31] GEORGE F O, SKELDON P, THOMPSON G E. Formation of zirconium-based conversion coatings on aluminium and Al-Cu alloys[J]. Corrosion Science, 2012, 65: 231-237.
[32] MOFFAT T P, LATANISION R M. An electrochemical and X-ray photoelectron spectroscopy study of the passive state of chromium[J]. Journal of The Electrochemical Society, 1992, 139(7): 1869-1879.
[33] QI J T, HASHIMOTO T, WALTON J R, ZHOU X, SKELDON P, THOMPSON G E. Trivalent chromium conversion coating formation on aluminium[J]. Surface and Coatings Technology, 2015, 280: 317-329.
[34] ALLEN G C, M.T. C, HOOPER A J, TUCKER P M. X-ray photoelectron spectroscopy of chromium-oxygen systems[J]. Journal of the Chemical Society, Dalton Transactions, 1973(16): 1675-1683.
[35] QI J, WALTON J, THOMPSON G E, ALBU S P, CARR J. Spectroscopic studies of chromium Ⅵ formed in the trivalent chromium conversion coatings on aluminum[J]. Journal of The Electrochemical Society, 2016, 163(7): C357-C363.
[36] SLEIGH C, PIJPERS A P, JASPERS A, COUSSENS B, MEIER R J. On the determination of atomic charge via ESCA including application to organometallics[J]. Journal of Electron Spectroscopy and Related Phenomena, 1996, 77(1): 41-57.
[37] STERN M, GEARY A L. Electrochemical polarization I. A theoretical analysis of the shape of polarization curves[J]. Journal of The Electrochemical Society, 1957, 104(1): 56-63.
[38] 钟丽应, 曹发和, 施彦彦, 文 强, 张 昭, 张鉴清. AZ91镁合金表面铈基稀土转化膜的制备及腐蚀电化学行为[J]. 金属学报, 2008, 44(8): 979-985.
ZHONG Li-ying, CAO Fa-he, SHI Yan-yan, WEN Qiang, ZHANG Zhao, ZHANG Jian-qing. Preparation and corrosion electrochemistry behavior of cerium-based chemical conversion coating on AZ91 magnesium alloy[J]. Acta Metallurgica Sinica, 2008, 44(8): 979-985.
[39] LIU W, LI Mou-cheng, LUO Q, FAN Hong-qiang, ZHANG, Jie-yu, LU, Hu-sheng, CHOU, Kuo-chih, WANG, Xun-li, LI Q. Influence of alloyed magnesium on the microstructure and long-term corrosion behavior of hot-dip Al-Zn-Si coating in NaCl solution[J]. Corrosion Science, 2016, 104: 217-226.
[40] ROSALBINO F, SCAVINO G, MORTARINO G, ANGELINI E, LUNAZZI G. EIS study on the corrosion performance of a Cr(Ⅲ)-based conversion coating on zinc galvanized steel for the automotive industry[J]. Journal of Solid State Electrochemistry, 2010, 15(4): 703-709.
[41] TOMACHUK C R, ELSNER C I, DI SARLI A R, FERRAZ O B. Corrosion resistance of Cr(Ⅲ) conversion treatments applied on electrogalvanised steel and subjected to chloride containing media[J]. Materials Chemistry and Physics, 2010, 119(1/2): 19-29.
[42] 顾宝珊, 杨培燕, 宫 丽. 电化学阻抗谱技术研究Ce(III)转化膜在3.5%NaCl溶液中的腐蚀行为[J]. 中国有色金属学报, 2013, 23(6): 1640-1647.
GU Bao-shan, YANG Pei-yan, GONG Li. Corrosion behavior of Ce(III) conversion coating in 3.5%NaCl solution by electrochemical impedance spectroscope[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(6): 1640-1647.
[43] 曹楚南, 张鉴清. 电化学阻抗谱导论[M]. 北京: 科学出版社, 2002: 151.
CAO Chu-nan, ZHANG Jian-qing. Introduction to electrochemical impedance spectroscopy[M]. Beijing: Science Press, 2002: 151.
[44] 余会成, 陈白珍, 石西昌, 李 兵, 吴海鹰. 6063铝合金三价铬化学转化膜的制备与电化学性能[J]. 物理化学学报, 2008, 24(8): 1465-1470.
YU Hui-cheng, CHEN Bai-zhen, SHI Xi-chang, LI Bing, WU hai-ying. Preparation and electrochemical properties of trivalent chromium coating on 6063 aluminium alloy[J]. Materials Chemistry and Physics, 2008, 24(8): 1465-1470.
[45] CAMPESTRINI P, VAN WESTING E P M, DE WIT J H W. Influence of surface preparation on performance of chromate conversion coatings on Alclad 2024 aluminium alloy Part Ⅱ: EIS investigation[J]. Electrochimica Acta, 2001, 46(17): 2631-2647.
[46] SINGH A, LIN Y, EBENSO E E, LIU W, PAN J, HUANG B. Gingko biloba fruit extract as an eco-friendly corrosion inhibitor for J55 steel in CO2 saturated 3.5% NaCl solution[J]. Journal of Industrial and Engineering Chemistry, 2015, 24: 219-228.
[47] 雷 黎, 王 昕, 徐海港. 镁合金铈转化膜在NaCl溶液中的腐蚀行为及腐蚀机理[J]. 中国有色金属学报, 2015, 25(1): 125-132.
LEI Li, WANG Xin, XU Hai-gang. Corrosion behavior and corrosion mechanism of cerium conversion coating on magnesium alloy in NaCl solution [J]. The Chinese Journal of Nonferrous Metals, 2015, 25(1): 125-132.
[48] LI Y. Formation of nano-crystalline corrosion products on Zn-Al alloy coating exposed to seawater[J]. Corrosion Science, 2001, 43(9): 1793-1800.
[49] 吴海江, 卢锦堂. 热镀锌钢表面铈转化膜的表征与腐蚀电化学行为[J]. 中国有色金属学报, 2011, 21(5): 1009-1015.
WU Hai-jiang, LU Jin-tang. Characterization and electrochemical corrosion behavior of cerium conversion coating on hot-dip galvanized steel [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(5): 1009-1015.
[50] DONG X, WANG P, ARGEKAR S, SCHAEFER D W. Structure and composition of trivalent chromium process (TCP) films on Al alloy[J]. Langmuir, 2010, 26(13): 10833-10841.
[51] XU Y, LI H, SHEN Y, LIU S, WANG W, TAO J. Improvement of adhesion performance between aluminum alloy sheet and epoxy based on anodizing technique[J]. International Journal of Adhesion and Adhesives, 2016, 70: 74-80.
[52] LI L, KIM D Y, SWAIN G M. Transient formation of chromate in trivalent chromium process (TCP) coatings on AA2024 as probed by Raman spectroscopy[J]. Journal of The Electrochemical Society, 2012, 159(8): C326-C333.
[53] JIANG Y, NI P, CHEN C, LU Y, YANG P, KONG B, FISHER A, WANG X. Selective electrochemical H2O2 production through two-electron oxygen electrochemistry[J]. Advanced Energy Materials, 2018, 8(31): 1-25.
[54] HESAMEDINI S, BUND A. Formation of Cr(Ⅵ) in cobalt containing Cr(Ⅲ)-based treatment solution[J]. Surface and Coatings Technology, 2018, 334: 444-449.
[55] XIA L, MCCREERY R L. Chemistry of a chromate conversion coating on aluminum alloy AA2024-T3 probed by vibrational spectroscopy[J]. Journal of the Electrochemical Society, 1998, 145(9): 3083-3089.
[56] MASLAR J E, HURST W S, BOWERS W J Jr, HENDRICKS J H, AQUINO M I, LEVIN I. In situ Raman spectroscopic investigation of chromiun surfaces under hydrothermal conditions[J]. Applied Surface Science, 2001, 180(1/2): 102-118.
[57] HURLEY B L, MCCREERY R L. Raman spectroscopy of monolayers formed from chromate corrosion inhibitor on copper surfaces[J]. Journal of The Electrochemical Society, 2003, 150(8): B367-B373.
[58] RAMSEY J D, MCCREERY R L. Raman microscopy of chromate interactions with corroding aluminum alloy 2024-T3[J]. Corrosion Science, 2004, 46(7): 1729-1739.
[59] RAMSEY J D, XIA L, KENDIG M W, McCREERY R L. Raman spectroscopic analysis of the speciation of dilute chromate solutions[J]. Corrosion Science, 2001, 43(8): 1557-1572.
[60] KERAMIDAS V G, WHITE W B. Raman scattering study of the crystallization and phase transformations of ZrO2[J]. Journal of the American Ceramic Society, 1974, 57(1): 22-24.
Construction of Cr(Ⅲ) chemical conversion coating on Zn-55%Al-1.6%Si assisted by pulse potential method
PAN Jie, TANG Xiao, LI Yan
(School of Materials Science and Engineering, China University of Petroleum (East China), Qingdao 266580, China)
Abstract: Cr(Ⅲ) chemical conversion passivation (TCP) exhibits a high potential as substitutes for the environmentally unfriendly chromate metal-surface pre-treatment methods. In this paper, Cr(Ⅲ) conversion coating was constructed on the surface of hot-dip Zn-55%Al-1.6%Si, and the structure and properties of the conversion coating were regulated by pulse potential method (PPM). 3D topography, SEM, XPS, Raman spectrum, contact angle, neutral salt spray test and electrochemical test were used to study the corresponding structure, composition and properties of the conversion coatings. The results of micro morphology and roughness analysis show that the surface of Cr(Ⅲ) conversion coating has the characteristics of multi-scale micro morphology, and nano-scale particles and holes are distributed on the micro-scale two-phase structure. The results also show that the application of external electric field reduces the micro-cracks and the content of hexavalent chromium, accelerated the formation of conversion coating, and the content of alkaline hydroxide in the coating increased as well. The contact angle measurement shows that the structure of conversion coating controlled by the pulse potential method has more certain hydrophobicity. When the pulse square wave potential φP=-100.0 mV, the TCP conversion coatings have the best corrosion resistance, which can improve the temporary protection of the hot-dip coating surface. The external pulse electric field has a positive regulatory and control effect.
Key words: hot-dip coated steel sheet; chemical conversion coating; trivalent chromium; pulse potential method; corrosion resistance
Foundation item: Projects(41676071, 51979282) supported by the National Natural Science Foundation of China; Project(18CX05021A) supported by the Fundamental Research Funds for the Central Universities, China
Received date: 2019-12-26; Accepted date: 2020-04-29
Corresponding author: LI Yan; Tel: +86-13615321199; E-mail: yanlee@upc.edu.cn
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(41676071,51979282);中央高校基本科研业务费专项资金资助项目(18CX05021A)
收稿日期:2019-12-26;修订日期:2020-04-29
通信作者:李 焰,教授,博士;电话:13615321199;E-mail:yanlee@upc.edu.cn
摘 要:采用化学转化法在Zn-55%Al-1.6%Si热镀层表面构建了三价铬转化膜,通过3D形貌仪、扫描电镜(SEM)、X射线光电子能谱(XPS)、拉曼光谱和接触角仪等表面分析手段,以及中性盐雾试验和电化学测试方法表征了转化膜的结构、组成和性能。利用脉冲电位法对转化膜的结构进行调控,研究了脉冲电场调控作用对转化膜结构和性能的影响。结果表明:构建的三价铬转化膜表面具有多尺度的微观形貌特征,微米级的两相结构上分布着纳米级颗粒和微孔。加载阴极脉冲方波可加速转化膜的生成,提高膜中氢氧化物的含量和膜的疏水性,还可减少膜表面的微裂纹以及Cr(Ⅵ)的含量;当脉冲方波电位φP=-100.0 mV时,构建的转化膜具有最佳的耐蚀性,能够提高对镀层表面的临时保护作用。外加脉冲电场对转化膜的结构和性能具有积极的调控作用。
[20] 袁训华, 林 源, 张启富. 热镀锌铝镁镀层的切边保护性能和耐腐蚀机理[J]. 中国有色金属学报, 2015, 25(9): 2453-2463.
[22] 范嘉雯, 程学群, 李晓刚, 肖 葵, 董超芳. 纯锌在我国热带海洋大气环境耐蚀寿命预测模型[J]. 中国有色金属学报, 2016, 26(4): 797-806.
[23] 章小鸽. 镀锌保护钢铁的效率和新型锌镀层的发展前景[J]. 中国腐蚀与防护学报, 2010, 30(2): 166-170.
[24] 李 焰, 邢少华, 李 鑫, 魏绪钧. 热浸镀层在青岛站的海水腐蚀行为对比(Ⅲ)—飞溅区[J]. 中国有色金属学报, 2007, 17(9): 1527-1535.
[38] 钟丽应, 曹发和, 施彦彦, 文 强, 张 昭, 张鉴清. AZ91镁合金表面铈基稀土转化膜的制备及腐蚀电化学行为[J]. 金属学报, 2008, 44(8): 979-985.
[42] 顾宝珊, 杨培燕, 宫 丽. 电化学阻抗谱技术研究Ce(III)转化膜在3.5%NaCl溶液中的腐蚀行为[J]. 中国有色金属学报, 2013, 23(6): 1640-1647.
[43] 曹楚南, 张鉴清. 电化学阻抗谱导论[M]. 北京: 科学出版社, 2002: 151.
[44] 余会成, 陈白珍, 石西昌, 李 兵, 吴海鹰. 6063铝合金三价铬化学转化膜的制备与电化学性能[J]. 物理化学学报, 2008, 24(8): 1465-1470.
[47] 雷 黎, 王 昕, 徐海港. 镁合金铈转化膜在NaCl溶液中的腐蚀行为及腐蚀机理[J]. 中国有色金属学报, 2015, 25(1): 125-132.
[49] 吴海江, 卢锦堂. 热镀锌钢表面铈转化膜的表征与腐蚀电化学行为[J]. 中国有色金属学报, 2011, 21(5): 1009-1015.


