
Integrated waste and water management in mining and metallurgical industries
B. K. C. CHAN1, S. BOUZALAKOS2, A. W. L. DUDENEY1
1. Department of Earth Science and Engineering, Imperial College, London SW7 2AZ, UK;
2. Centre for Innovation in Carbon Capture and Storage (CICCS), University of Nottingham,
Nottingham NG7 2RD, UK
Received 20 September 2008; accepted 5 November 2008
Abstract:
Extractive operations usually co-produce large quantities of unmarketable materials (mineral wastes), most of which are conventionally discarded to dumps (coarse material) and tailings ponds (fines). Escalating cost and regulation worldwide highlight an increasing need for reduction and re-use of such wastes. The present paper introduces a new integrated waste management scheme for solids and water. The scheme was exemplified by novel treatment of synthetic waste and process water linked to the biohydrometallurgical processing of metal sulphide flotation concentrates. Bioleaching of sulphide concentrate leads to two types of solid waste: a ferrihydrite/gypsum precipitate from neutralisation of the bioleach liquor and un-leached gangue. The paper indicates that, depending upon the minor components involved, the solid phases in admixture might be usefully distributed among three types of product: conventional underground backfill, cemented civil engineering backfill (particularly controlled low strength material or CLSM) and manufactured soil. It emphasizes CLSM containing simulated mineral waste, showing that such material can exhibit the required characteristics of strength, porosity and permeability. When toxic components, e.g., arsenic from refractory gold ore, are present, encapsulation will be required. Process water is typically recycled as far as possible, although any excess should be treated before re-use or discharge. The paper also highlights treatment by reverse osmosis (one of the few methods able to generally remove dissolved components), particularly showing that arsenic in oxidation state +6 can be readily removed for discharge (<50×10-12 As), although additional ion exchange is needed for potable water (<10×10-12 As).
Key words:
cementation; waste processing; bioleaching; tailings; refractory gold; arsenic; controlled low strength materials; reverse osmosis;
1 Introduction
Extractive operations, i.e. exploration, mining, mineral and metallurgical processes, which are employed internationally for the provision of primary and secondary metal and mineral commodities, usually co-produce large quantities of unmarketable or uneconomic materials[1]. These wastes, which may be a major source of pollution, include mining waste (topsoil, overburden and waste rock), processing waste (collectively referred to as tailings), and metallurgical waste (slag, flue dust, leach residues and precipitates) [1-2]. In accordance with the European Mine Waste Directive[3] and ‘best available technology’ BAT, typically 30%-50% of these wastes are back-filled in mining voids. The remainder of the materials is conventionally discarded, at significant cost, to engineered structures such as mineral dumps for coarse material and tailings dams for fine material. However, increasing emphasis is placed on re-utilisation, rather than storage/disposal, of mine waste for the future through innovative solutions and emerging technologies due to increasing cost of disposal and stringent environmental regulation. Changes are slow, with conservative mine operators continuing to focus on conventional ‘good practice’ guidelines. Nonetheless, new opportunities for reduction and re-use of mineral waste are becoming possible. In particular, combinations of mineral wastes with other bulk industrial products, such as power station ash, can be assessed for application in civil engineering.
This paper deals with a new approach to integrated waste management in which all significant products of metal production are linked and all are in principle utilised. The approach is designed to general applicability, but, of course, the details will differ greatly from one process to another. In the present work, the approach has been exemplified through bioleaching of sulphide flotation concentrates[4-5]. As process water and waste solid were not available from operating plant, synthetic analogues were studied.
2 Theory and objectives
Fig.1 gives a scheme[6] from which the principles of integrated waste management can be seen. The scheme is meant to have general applicability, although the details would obviously differ greatly in each application. The figure shows plausible inputs from a typical mineral processing operation, proportions for water recycle and examples of the re-use of products, variously destined for mine backfill, agricultural (or restoration) soil and civil engineering construction. According to the scheme, the inputs of solids and water are partially separated by dewatering and decantation to facilitate water recycle and/or discharge (some 70%-80% of the total water in the system) and to increase the solids content of waste to about 70%-80% by mass (40%-50% by volume). The resulting solids, containing some 20%-30% by mass of water, are mixed with selected products from other industries, as exemplified by mineral matter (cement, cement kiln dust, lime metallurgical slag, waste gypsum, power station ash and/or incinerator ash) for engineering products and organics (various formulations of sewage sludge, e.g., anaerobically digested sludge with green waste) for soils. Contaminated soil and subsoil from former industrial sites might also be of interest. Representative compositions are given in the scheme for formulated soils, and cemented products—viscous pastefill for stabilising voids and ‘flowable’ controlled low strength materials for groundwork construction. Gaseous emissions and losses of heat to the surroundings are not considered in the figure. They are likely to be relatively small for unit operations in waste management (even though upstream energy inputs and losses can be quite large, e.g., in ore crushing and grinding) because most relevant processes occur in the condensed phase at or near ambient temperature. Gaseous emissions and energy losses associated with the production of sewage products, cement, coal fired power station ash, and related products may also be substantial.
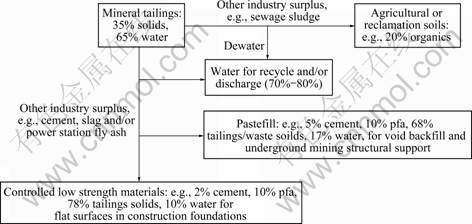
Fig.1 Scheme of integrated waste management
Aspects of the scheme are already common practice in the industry, particularly mine backfill mentioned above, and surface restoration, perhaps as mine spoil amended with lime and sewage products to form a growing medium for plants. However, a far greater volume of solid is generally produced than can be applied in these ways in the vicinity of a mining operation. Thus, backfill are limited by the increased volume of comminuted products and by inaccessibility, e.g., because of subsidence, while surface spreading and soil amendment is limited by available area near a mine. To utilise a greater proportion of the solid, artificial soil products containing mineral waste (which would be transportable to remote markets), might also be formulated with specially treated sewage products[7]. However, this application remains in its infancy. Another approach is to formulate cemented products incorporating waste for the construction industry, particularly for applications in building and perimeter foundations requiring low loading capacity, within, say, a 10 mile radius of a mining operation. Thus, so-called controlled low strength materials(CLSM), containing sand, cement and pulverised fuel ash, have been developed in recent years in civil engineering, but not so far for dealing with mineral process waste. One objective of the present work was to test relevant characteristics, particularly compressive strength, porosity and permeability, of CLSM containing such waste.
Metal extraction from mineral process concentrates,e.g., metalliferous sulphide flotation concentrates, should be especially suited to the application of IWM. Thus, upstream processes of comminution and concentration produce particle sizes suitable for flowable cemented products (or soils), together with considerably reduced bulk in comparison with the original ore. However, the process water and solid waste produced are likely to be complex and may contain significant concentrations of toxic elements, e.g., As. Safe management is necessary, including effective removal of deleterious contaminants, when necessary, from process water together with effective immobilisation or containment of them in the solid cemented products. A second objective was therefore to investigate purification of process water by reverse osmosis (one of the few techniques able to remove dissolved species) and to test the stability of solid products against leaching under environmental conditions.
Fig.2 gives a generalised flowsheet of basic relationships between direct bioleaching, water management and waste treatment in proposed options[6] for the production of base metals (especially copper and/or nickel) and gold from sulphide flotation concentrates. The figure indicates the options for water purification and integrated waste management. For clarity, it excludes details of inputs, e.g., the composition of the bioleach pulp, configurations of unit operations and the compositions of outputs. The general term ‘extraction’ is used to represent the configurations of particular processes, e.g., selective precipitation (Ni), solvent extraction/electrowinning (Cu) and cyanidation (Au). The products (designated Cu/Ni and Au) are variable depending upon details of process design. Possibilities are refined copper cathode, precipitated nickel hydroxide and impure gold (the Ni and Au products to be refined at a smelter).
Regarding water management, the flowsheet shows alternative possibilities of total water recycle or partial recycle and ‘make-up’, with or without integral purification linked to amenity use or discharge. Water make-up is relevant for a process with a negative water balance, e.g., less water from sedimentation and recycling than needed in the process. Discharge or amenity use are options when the balance is positive (more available than required for recycling to the process). Water purification may then be appropriate. Chemical precipitation, filtration and reverse osmosis are examples of unit operations used in such purification.
Long-established practice is thus employed through dewatering and recycling water within the process, as far as feasible. A traditional dewatering route is by gravity settlement and decantation using a tailings pond, although mechanical centrifuging and/or filtration might be employed. Water balance and pulp density (proportion of solid to water) vary substantially from one mineral process, and from one part of a process, to another. However, the water contents and settled densities are relatively steady in tailings ponds. The water content in settled solids, although much lower than in pulp, is still likely to be substantial and may be in excess of that needed in integrated waste management. Further water removal is thus indicated (Fig.2).
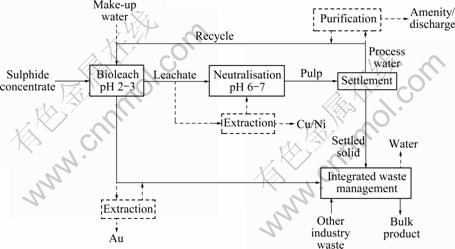
Fig.2 Generalised flowsheet of metals bioleach, water management and waste treatment processes
The figure also shows the formation of two main types of solid residue: a ferrihydrite/gypsum-rich precipitate from limestone neutralisation of barren bioleach liquor and a mineral-rich residue from bioleaching. Both might be modified by different metal extraction procedures (mainly solvent extraction/ electrowinning for copper, carbonate or hydroxide precipitation for nickel and cyanidation/carbon absorption for gold,). The waste solids would ideally be treated together with imported local materials (other industry waste) to yield a marketable bulk product. The flowsheet shows the link to IWM, apparently as an essentially a ‘pipe end’ procedure. However, the various bioleach and waste management processes are likely to be mutually interactive to a greater or lesser extent and therefore subject to optimisation to achieve the best overall technical and economic outcome.
For instance, settlement conditions are designed to take account of particle size effects: bioleaching benefits from fine particle sizes (through fine grinding) while separation of solid from water (through gravity sedimentation) is more efficient with larger particles, with consequent effects on water content going through to waste treatment. Mineralogy is also an important consideration: some minerals, e.g., silica sand and many aluminosilicates, form individual free-settling particles, while finer-sized hydrous oxides, like ferrihydrite, may sediment very slowly. Sedimentation may be further retarded by solids forming colloid or gel structures in water, e.g., montmorillonite and other smectite phases. Such variables can be optimised using material balances for different operating conditions, once detailed site-related feasibility studies have been carried out.
3 Experimental
3.1 Controlled low strength materials
CLSM were characterised by very high workability, low density, and strength[8-10], having a flowable and self-levelling consistency[11-13]. They are typically a blend of portland cement (PC), pulverised fuel ash (PFA), fine/coarse aggregates and water; that upon hydration of the cementitious and pozzolanic material produces a solidified geotechnical composite suitable for fill applications[9]. A minimum compressive strength of 0.44 MPa (walkability limit) should be achieved in order to be excavatable by mechanical equipment[14] and maximum design strength of 2 MPa (excavatable limit) after 28 d of curing should also be obtained to provide sufficient support for construction and vehicle loads.
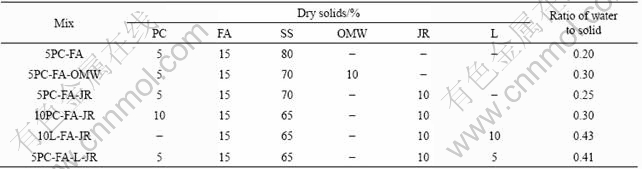
The model wastes chosen to represent the neutralisation precipitates were an ochreous mine water waste (OMW-fine-grained, Fe- and Ca-rich neutralisation precipitates from bioleaching with relatively low levels of hazardous components) and an industrial jarosite residue (JR—with higher levels of hazardous components). Silica sand(SS) was used as the bulk mineral material in the CLSM formulations. A commercially available cement(PC), PFA and Lime(L) were used as binder.
A conventional mix, 5PC-FA, was initially mixed with a mechanical stirrer, deionised water was added gradually until the mix gave a spread diameter of (229± 10) mm. Further mixing was carried out until the mix had a uniform consistency and appearance and gives a compressive strength within the excavatable and walkable limits of 2 and 0.4 MPa respectively. OMW and JR were introduced into the formulation of 5PC-FA by substituting fixed proportions of SS with waste. Table 1 lists the formulation for CLSM mix design.
Table 1 Material formulation for CLSM mix design
The mix was poured into cylindrical moulds of appropriate dimensions, depending upon the type of test to be performed. Due to the flowable nature of CLSM, no compaction or vibration was necessary during casting. Specimens were allowed to harden for about 3 d before mechanically de-moulding. Following de-moulding, specimens were cured in sealed plastic bags at room temperature until required for testing after 7, 14 and 28 d of curing.
The aim of this work was to investigate the laboratory-scaled CLSM specimens made from the above materials for physical (hydraulic conductivity & porosity), mechanical (unconfined compressive strength), and leaching properties (ICP-AES) of the waste materials.
For mechanical characterisation, triplicate cylindrical CLSM specimens for each mix design were subjected to unconfined compressive strength (UCS) testing after different curing periods. For physical characterisation, Porosity was evaluated using a helium pycnometer according to BS ISO 11599 (1997) [15]. Hydraulic conductivity (K) was determined using a high-pressure permeameter, particularly suitable for cement-based materials[16]. Prior to testing, porosity specimens were dried in an oven at 40 ℃ in order to avoid, as much as possible, internal cracking and shrinkage. Hydraulic conductivity specimens were vacuum saturated in de-ionised water for 4 h. For leaching characterisation, specimens were assessed using the Dutch diffusion leach test, commonly known as the ‘tank test’, in accordance to EA NEN 7375 (2005)[17]. Duplicate monolithic cylindrical specimens, for each mix design, cured for 28 d, were submerged into closed polyethylene beakers containing a leachant (de-ionised water with electrical conductivity of 61 μS/cm). The diffusion-leaching test was carried out for eight successive steps of specified length: 0.25, 1, 2.25, 4, 9, 16, 36 and 64 d. pH and electrical conductivity were monitored for all eight periods. Eluates were preserved immediately after filtration (0.45 μm) and collection by acidifying with HNO3 to pH<2. Chemical elemental analysis by ICP-AES (Varian VISTA PRO) of the waste materials and leach eluates, was undertaken at the Natural History Museum, London. Detailed description of specimen preparation, mixed formulation, characterisation and analytical techniques can be found by BOUZALAKOS et al[4, 18].
3.2 Purified water
This second part of the paper deals with the quality of liquid streams likely to arise from bioleaching of gold-bearing arsenical sulphide flotation concentrate with purification of effluent after lime neutralisation. The refractory gold flotation concentrates contain high levels of arsenate, which is dissolved during bioleaching and largely co-precipitated with ferrihydrite and gypsum during neutralisation of the leach liquors. The work is primarily relevant to processes having a positive water balance, e.g. raw water to a settling pond, or where a proportion of purified water is required for other purposes on a site, e.g. washing solids. It considers RO management of residual concentrations remaining after the co-precipitation (<0.1 mg/L As) and combined reverse osmosis and/or resin ion exchange (IX) treatment under designed conditions in a re-circulating system based on equipment provided by Purite Ltd.
Detailed experimental procedure can be found by CHAN et al[5]. Fig.3 shows the equipment constructed employing components provided by Purite Ltd. It includes units for reverse osmosis (TFM-100 with spiral-wound polyamide thin film composite membrane), micro-filtration (Hytrex cardridge filter) and ion exchange (D340 mixed bed resin). The equipment was designed to facilitate continuous recirculation of RO reject via the stock tank using a Purite custom-built pump system, and recovery of permeate for further treatment by ion exchange, as required. The pump typically generated 3 L/min flow at 0.4-0.6 MPa, controlled by a drain flow restrictor valve. Gypsum precipitated in the reject was recovered by in-line filtration at 1 or 5 mm.
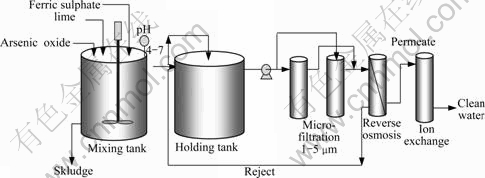
Fig.3 Equipment for reagent mixing, reverse osmosis, microfiltration and ion exchange
Test solutions were re-circulated in the equipment as follows. Neutralised filtrate was pumped from the holding tank to the microfiltration and RO units at pre-set pressure while membrane reject was returned to the holding tank. The process was continued until the volume remaining was too small (about 2 L after 3-4 h). Permeate was collected in approximately 2 L volumes over 30 min intervals. Samples of reject (500 mL) were taken at hourly intervals. When required, combined permeate volumes (10-14 L) were passed once-through the ion exchange column for further purification. Monitoring during these processes was carried out with hand-held probes for pH (WTW pH 330i) and conductivity (WTW conductivity 330i). Both instruments gave temperature measurement. Samples (approximately 1 L from each cycle) were retained for ICP elemental analysis (ICP-AES and ICP-MS) for calcium, sulphur, arsenic and other elements arising from impurities in reagents. Precipitated gypsum was recovered from the filter cartridge and holding tank. Residues were removed by flushing the equipment with water and/or a propriety purite anti-fouling solution. The distribution of arsenic at low concentrations required by potable water and groundwater regulations was determined by ICP-MS and ICP-AES.
4 Results and discussion
4.1 Cementation of waste
Fig.4 shows the typical variations of unconfined compressive strength (UCS) in cemented mixtures with specimen age (7, 14 and 28 d). All exhibited increasing strength with age, as expected for formulations containing cementitious binders-Portland cement (PC), fly ash (FA) and/or lime (L)-in a matrix of silica sand (SS). Specimens containing 5%, 15% and 80% PC, FA and SS, respectively, gave UCS of 0.5-1.5 MPa, satisfactorily within the limits 0.4-2.0 MPa published for civil engineering CLSM. Replacement of 10% of the sand with neutral ochreous waste resulted in similar strength. However, replacement with acidic jarosite waste resulted in mechanically weak composites having a tendency to disrupt in water, even with 10% binder, unless both cement and lime were used to neutralise the acidity.
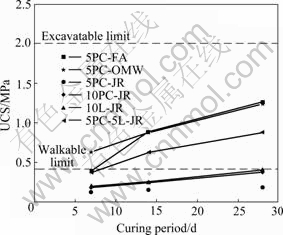
Fig.4 UCS of CLSM formulations at different curing periods
The porosity of specimens was in the range of 39%-44%, decreasing by about 6% over 7-28 d as crystallization occurred within pores. This behaviour was typical of CLSM. Hydraulic conductivity was (0.5-3.0)×10-6 m/s, similar to CLSM in analogous studies[13,19] and in the range expected for granular fills. Examples are shown in Fig.5. Hydraulic conductivity decreased with addition of waste ochre and jarosite because of their content of fine sized particles, which in-filled cavities in the specimens. Thus, although permeability was substantial in all cases, this was reduced with waste present (leading in principle to reduced loss of contaminants to the environment).
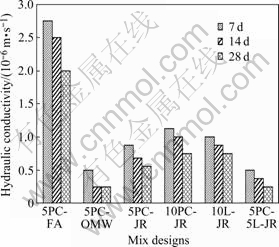
Fig.5 Hydraulic conductivity of CLSM formulations at different curing periods
Actual leachability was compared with groundwater intervention levels for contaminated land[20]. As expected, leachate from specimens immersed in water under standard conditions was alkaline and contained substantial levels of calcium sulphate. Examples of cumulative concentrations of other species are given in Fig.6. Concentrations of the heavy elements As, Cd, Co, Cu, Mn, Mo and Ni were below guideline values, even from specimens containing jarosite waste having elevated levels of these elements. Adsorption onto hydrated iron(Ⅲ) oxide abundant in the specimens accounted for the low mobility of arsenate. The metals should also be adsorbed, aided in most cases by low solubility at the high pH prevalent in lime and cement. However, cationic Ba exceeded guidelines for all specimens (including the cement/fly ash control) and the amphoteric Cr, Pb and Zn gave excessive concentrations for some formulations, particularly with jarosite.
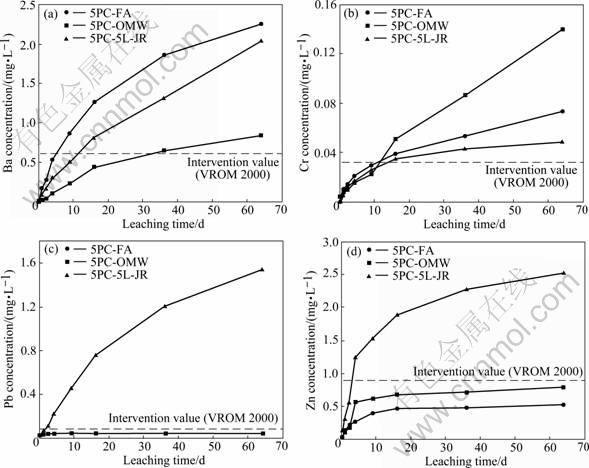
Fig.6 Tank leaching test of CLSM formulations over 64 d
From the results, formulations without significant concentrations of toxic elements from fly ash and/or mineral waste can provide credible CLSM. However, in other cases, development of a suitable containment system will be required before cemented mixtures can be promoted for use in the environment. Although detailed research is still required, it is clear that two types of containment can be described: particle and monolith. Thus, particles can be coated with a clean low permeability layer, such as magnesium carbonate, and large blocks of material can be contained within a lining, in a similar way to conventional landfill. The difference from such landfill is, of course, that the ground becomes immediately stable for use in civil engineering applications such as foundations for roads, car parks and low-rise buildings. Technical and regulatory hurdles need to be overcome.
4.2 Water purification
Preliminary work showed that neutralisation of synthetic liquor from bioleaching of gold-bearing pyrite/arsenopyrite concentrate gave 0.03-0.09 mg/L As in filtered neutralised leachate-similar to, or in excess of, guidelines for discharge. Reverse osmosis results involving arsenic at low concentration (Fig.7) in such filtrate show more or less constant arsenic in permeate (0.002-0.003 mg/L As) from successive cycles while concentrations steadily increased (0.03-0.13 mg/L As) in the diminishing volume of reject (separation factor about 12). Arsenic was in oxidation state +6, i.e., as arsenate. At the same time, concentrations of calcium and sulphate (not shown) remained low in permeate (<10 mg/L) while increasing to saturation (approximately 2.1 g/L CaSO4?2H2O) in the reject, with excess precipitated in mineral form. Very high initial concentrations of arsenic (2.9 mg/L As) were reduced to <100 mg/L As in one RO pass, with calcium and sulphate behaving as above. However, when arsenic was in partially or fully reduced state, i.e., much less complete separation was achieved because of the neutral characteristics of arsenite. Full oxidation was necessary.
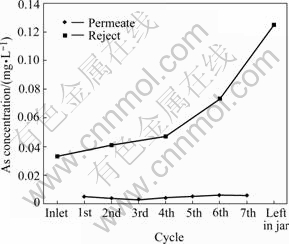
Fig.7 Concentration of arsenic in reject and permeate with initial arsenic concentration of 0.03 mg/L
Thus, arsenic levels were readily reduced to discharge standards and the proportion of water going to discharge could be varied widely to suit the water balance in a particular flowsheet (Fig.2). Conversely,arsenic in the reject stream can be recycled to neutralisation and partially adsorbed on precipitating ferrihydrite, i.e., can join the solid waste stream, with the filtered neutralised leachate again reaching about 0.03 mg/L As. As there is currently no evidence that moderately elevated arsenic levels adversely affect bioleaching, it may also be possible to employ As-enriched RO reject as make-up water ahead of bioleaching.
In principle, the gypsum precipitate might be marketed for use in plasterboard. However, it precipitates from the RO system in a lath-like crystalline habit unsuited for this purpose. Gypsum also co-precipitates with about one third of the dissolved arsenic. Therefore, it should join the solid waste stream, and may possibly be of benefit there for its cementitious properties. The removal of gypsum from water may also help to meet discharge criteria, as discharge consents for sulphate can be as low as 0.4 g/L SO42- (0.7 g/L CaSO4?2H2O) in EU countries.
Other metals, not considered in detail here were also greatly reduced in permeate by RO, and were potentially recyclable via the RO reject. Depending upon concentrations, they may be recoverable (cf Fig.2), or, as in the case of gypsum, simply prevent build-up of contaminants in discharged water.
5 Conclusions
1) The present work outlined a scheme of integrated waste management and exemplified a relatively little-studied aspect (controlled low strength materials containing mineral process waste) of it through determinations of important physicochemical criteria in comparison with controls and published guidelines. Thus, it was shown through measurements of unconfined compressive strength, porosity and hydraulic conductivity that materials can be formulated satisfactorily containing a 10% proportion of waste. However, further work is required to increase the proportion of waste utilised and, by suitable containment, to prevent leaching of significant concentrations of toxic elements from the materials. Once technically sound materials are available, sustainability grounds can be used to argue for regulatory acceptance.
2) The work also indicated how the process water could be purified by reverse osmosis for re-use, with contaminants being returned to, and integrated with, the main process flowsheet. Thus, conditions were outlined to show how dissolved and precipitated contaminants might join the solid waste stream.
Acknowledgements
This work was carried out in the frame of BioMine (Biotechnologies for metal bearing materials in Europe) (European project contract NMP1-CT-500329-1). The authors acknowledge the financial support given to this project by the European Commission under the Sixth Framework Programme on the development and integration of innovative, environmentally friendly, biohydrometallurgical processes such as bioleaching for the recovery of metals from primary and secondary resources within Europe (http://biomine.brgm.fr). We also wish to thank our various partners for their contributions Mintek, South Africa (John Neal) for supplying data on bioleach liquor; Umicore, Belgium, DRAX Power Station, UK and the Coal Authority, UK for supplying the materials; staff at Purite Ltd (particularly David Gray) for RO; Natural History Museum (Sarah James) for ICP-MS and Imperial College (Barry Coles for ICP-AES and Graham Nash for general technical assistance). Thanks also due to Emily Riddiford, Irina Tarasova and Rosie Davy for contributions to experimental procedures.
References
[1] Lottermoser B G. Mine wastes: Characterisation, treatment and environmental impacts [M]. Berlin Heidelberg, New York: Springer, 2003.
[2] BRGM. Management of mining, quarrying and ore-processing waste in the European Union [DB/CD], 2001.
[3] European Commission. Mining Waste: Management of Waste from the Extractive Industries [R]. Directive 2006/21/EC, 2006, EU Brussels.
[4] Bouzalakos S, Dudeney A W L, Cheeseman C R. Controlled low-strength materials containing waste precipitates from mineral processing [J]. Minerals Engineering, 2008, 21: 252-263.
[5] CHAN B K C, DUDENEY A W L. Reverse osmosis removal of arsenic residues from bioleaching of refractory gold concentrates [J]. Minerals Engineering, 2008, 21: 272-278.
[6] CHAN B K C, DUDENEY A W L, BOUZALAKOS S. State of the Art of Management of Wastewater and Tailings in Processes relevant to BioMinE [EB/OL]. http://biomine.brgm.fr/Documents/4BioMinE- Products/Deliverable/Report/BioMinE_DI8_Report.pdf, 2008.
[7] TAYLOR T J. Sludge phyto-conditioning: low technology enhanced treatment [J]. Water and the Environmental Journal, 2008, 18: 196-201.
[8] Nataraja M C, Nalanda Y. Performance of industrial by-products in controlled low-strength materials (CLSM) [J]. Waste Management, 2007, 28: 1168-1181.
[9] Al-Harthy A S, Taha R, Abu-Ashour J, Al-Jabri K, Al-Oraimi S. Effect of water quality on the strength of flowable fill mixtures [J]. Cement & Concrete Composites, 2005, 27: 33-39.
[10] Du L, Folliard K J, Trejo D. Effects of constituent materials and quantities on water demand and compressive strength of controlled low-strength material [J]. Journal of Materials in Civil Engineering, 2002, 14: 485-495.
[11] Taha R, Al-Rawas A, Al-Jabri K, Al-Harthy A, Hassan H, Al-Oraimi S. An overview of waste materials recycling in the sultanate of Oman [J]. Resources Conservation & Recycling, 2004, 41: 293-306.
[12] Dockter B A. Comparison of dry scrubber and class C fly ash in controlled low-strength material (CLSM) applications [C]// Proceedings of Design and Application of Controlled Low-Strength Materials (Flowable Fill). Missouri: ASTM STP 1331, St. Louis, 1998: 13-26.
[13] ACI (American Concrete Institute). Controlled low-strength materials (CLSM) [J]. Concrete International, 1994, 16: 55-64.
[14] Gabr M A, Bowders J J. Controlled low-strength material using fly ash and AMD sludge [J]. Journal of Hazardous Materials, 2000, 76(2/3): 251-263.
[15] BS ISO 11599. Determination of gas porosity and gas permeability of hydraulic binders containing embedded radioactive waste [S]. UK: BSI, 1997.
[16] Green K M, Hoff W D, Carter M A, Wilson M A, Hyatt J P. A high pressure permeameter for the measurement of liquid conductivity of porous construction materials [J]. Review of Scientific Instruments, 1999, 70 (8): 3397-3401.
[17] Leaching characteristics of granular building and waste materials. The determination of the availability of inorganic components for leaching: The tank test [R]. EA NEN 7375. UK: Environment Agency, 2005.
[18] Bouzalakos S, Dudeney A W L, Cheeseman C R. CLSM with solid wastes from processing of metal bearing resources [C]// Proceedings of 2nd International Conference on Advances in Mineral Resources Management and Environmental Geotechnology (AMIREG). Greece: Hania, Crete, 2006: 293-298.
[19] Naik T R, Singh S S, Ramme B W. Performance and leaching assessment of flowable slurry [J]. Journal of Environmental Engineering (ASCE), 2001, 127(4): 359-368.
[20] The Ministry of Housing, Spatial Planning and Environment, Netherlands. Intervention values and target values—soil quality standards, Department of Soil Protection, the Haque, Netherlands, 1994.
Corresponding author: B. K. C. CHAN; Tel: +44-20-7594-7435; E-mail: bkc.chan@imperital.ac.uk
(Edited by LI Xiang-qun)


