
Megahertz magneto-dielectric properties of nanosized NiZnCo ferrite
from CTAB-assisted hydrothermal process
SHEN Xiang(沈 翔)1, 2, WANG Yan-xin(王焰新)3, YANG Xiang(杨 祥)1, 2,
XIA Yong(夏 勇)2, ZHUANG Jian-feng(庄剑锋)2, TANG Pei-duo(唐培朵)2
1. Engineering Research Center of Nano-Geomaterials of Ministry of Education,
China University of Geosciences, Wuhan 430074, China;
2. Faculty of Material Science and Chemical Engineering, China University of Geosciences, Wuhan 430074, China;
3. School of Environmental Studies, China University of Geosciences, Wuhan 430074, China
Received 10 August 2009; accepted 15 September 2009
Abstract:
A simple hydrothermal route with cetyltrimethyl ammonium bromide (CTAB) was proposed for directly synthesizing single-crystalline NiZn ferrite at 160 ℃. X-ray diffraction patterns and micrographs indicate that products consist of spinel ferrite nanogranulars. The dielectric constant of NiZnCo ferrite is about 11 and the imaginary part of complex permittivity is 1.3. The saturation magnetization (Ms) of NiZn ferrite improves from 0.041 to 0.074 A·m2/g for Ni0.49Zn0.5Co0.01Fe1.98O4. The dielectric contant of NiZn ferrite increases from 7 to 11 with a cobalt stoichiometry of 0.01. The real part μ′ of complex permeability for NiZnCo ferrite reaches 3 at 1 GHz. The imaginary part μ″ of NiZnCo ferrite has a value higher than 1.2 within 0.7-3.0 GHz. Through the incorporation of the magnetic fillers, the low dielectric constant of the composites may meet the requirements of impedance matching to achieve the maximal absorption of the electromagnetic energy in megahertz frequency range.
Key words:
NiZnCo ferrite; hydrothermal; permeability; permittivity; saturation magnetization;
1 Introduction
Soft magnetic NiZn ferrite nanoparticles have critical need for the very high frequency applications such as broadband transformers, filters, antennas and microwave absorbing materials[1-2]. The control of the dielectric behaviors as well as the magnetic properties at hyper-frequencies has attracted much attention in recent years[3]. Since the electromagnetic properties of spinel ferrites are sensitive to their compositions and microstructures[4], the key to obtaining high- performance ferrites is a synthesis by a special technique. Traditional ceramic preparation methods[5-6] for nanosized spinel ferrite often suffer from uncontrolled stoichiometric composition, contamination and high annealing temperature[7-8]. The hydrothermal technology[9] is very versatile for the synthesis of nanophase materials, owing to its economics and the high degree of compositional control[10]. Moreover, it is green because reactions occurred in closed systems at low temperatures of about 160 ℃. As Ni is expected to couple ferromagnetically with Fe, we are interested in studying the effect of substitution of Fe with Ni in Ni0.5Zn0.5Fe2O4, particularly on the dynamic electromagnetic parameters. Meanwhile, we substitute the small amount of magnetic anisotropic cobalt[11] into NiZn ferrite to control their magnetic characteristics such as Ms, coercive force (Hc), and high frequency permeability, μ′ and μ″. In the past few years, we have worked on the synthesis of soft magnetic NiZnCo ferrite thin film with nanogranulars from thermal solution[12]. The ferrite films with high resistivity and high permeability can be applied to suppressing electromagnetic interference. Recently, we are absorbed in synthesizing nanocrystalline NiZnCo ferrite from hydrothermal process. The impetus for this is that the nanosized ferrites present novel physic properties different from their bulk counterpart.
In this work, a simple hydrothermal route, using CTAB as surfactant, was proposed for synthesizing single-crystalline NiZnCo ferrite nanoparticles at low temperature. The influence of the preparation techniques as well as the structural doping of Co and Ni ions on the dynamic magneto-dielectric properties of the compound was investigated. The work is intended to improve the soft magnetic properties and the permeability of NiZn ferrite with the small cobalt amount.
2 Experimental
The purities of all the reagents used were of analytical grade without further purification. Nanoparticles of (NixZn1-x)CoyFe2-yO4 (x=0, 0.4, 0.5, 0.6; y=0, 0.01) were synthesized by the hydrothermal route. In a typical preparing procedure, cetyltrimethl ammonium bromide (CTAB) and 1 g of urea were dissolved in 40 mL deionized water to form a transparent solution. The urea was as a homogeneous precipitation agent. Then ferrous ammonium sulphate of 1.6 g was added to the solution. After stirring for 10 min, stoichiometric amount of Ni(NO3)2·6H2O, Zn(NO3)2·6H2O and Co(NO3)2·6H2O was introduced into the mixed solution under vigorous stirring. The deionized water was added to make the total solution volume of 80 mL, and pH of the solution was adjusted to 7 with dilute ammonia. Before being transferred to a Teflon-lined autoclave of 100 mL capacity, the solution mixture was pretreated under an ultrasonic water bath for 20 min. Hydrothermal synthesis was carried out at 160 ℃ for 6 h in an electric blowing dry oven and cooled to room temperature. The collected brown precipitate was washed with distilled water and alcohol to remove any possible impurities. The produced ferrites were dried at 110 ℃ under vacuum for 12 h.
The prepared samples were analyzed by transmission electron microscope (TEM, FEI-Tecnai G220), atomic force microscope (AFM, MultiMode/ NS3A), hysteresis loops measurements via vibrating sample magnetometer (TM-VSM2050HGC). The phase structure of the powder was identified using an X-ray diffractometer (X’Pert PRO DY2198) with a Cu target at 40 kV and 40 mA. The X-ray diffraction (XRD) data were recorded via a 2θ angular increment of 0.017? and a scan step time of 9.25 s. The frequency variations of the permeability and the permittivity were measured using an Agilent 8722ES vector network analyzer on polycrystalline toroidal samples, in a coaxial wave guide between 0.3 and 3 GHz, at room temperature[13]. The test samples with the dimension of 7 mm×3 mm×3.3 mm were made of prepared ferrites and non-magnetic paraffin with the ferrite mass fraction of 80%.
3 Results and discussion
Fig.1 indicates the XRD patterns of prepared samples by hydrothermal treatment in the presence of CTAB. All the XRD peaks can be indexed to the cubic spinel structure with no extra lines corresponding to any other phases, which shows that the prepared ferrites are single phase. The broadened nature of these diffraction peaks indicates that the grain sizes of the samples are on nanometer scale. The mean crystallite size was calculated from the XRD line width of (311) peak using Scherrer formula to be 10-40 nm (Table 1). The overall chemical composition for the as-prepared samples was determined by energy dispersion X-ray analyzer. It is noticed that the crystallite size of doped zinc ferrite with Ni2+ or Co2+ ion is larger than 8.6 nm of zinc ferrite, indicating that the size of the particles augments when Zn2+ is replaced with Ni2+.
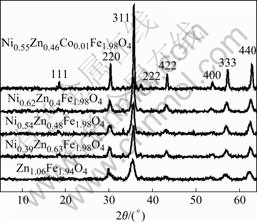
Fig.1 XRD patterns of prepared ferrite samples via hydrothermal route at 160 ℃ for 6 h
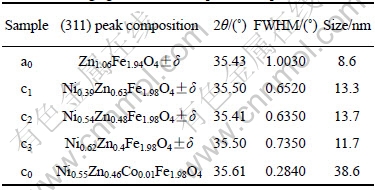
Table 1 Average granular sizes of produced spinal ferrites
The cationic surfactant generally adsorbs onto the interface with its positively charged hydrophilic head group oriented toward the negatively charged surface and its hydrophobic group oriented away from the surface, making the surface water-repellent[14]. The longer length of the hydrophobic group for CTAB causes closer packing of the surfactant molecules at the interface and increases the tendency of the surfactant to adsorb at an interface or to form micelles. The free energy of the solution can be decreased by the aggregation of the surface-active molecules into micelles with their hydrophobic groups directed toward the interior of the cluster and their hydrophilic groups directed toward the solvent. The nucleation process is inside the micelles and the aggregation process forms the final particle. The size of the final particles is controlled by the surrounding surfactant molecules. The increase in concentration of CTAB may cause change in the crystalline size from 38, 22 to 62 nm (Fig.2), showing that the crystalline size for critical micelle concentration (CMC, 8.5 mmol/L) is smaller. With the increase of aggregation number of the micelle, the shape of particles varies from spherical to rod (Fig.3(a)).
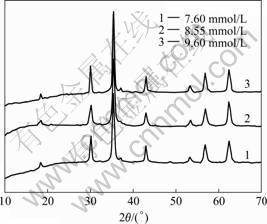
Fig.2 XRD patterns of NiZnCo ferrite prepared by hydrothermal process at 160 ℃ in different concentrations of CTAB
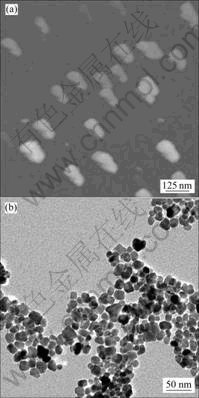
Fig.3 AFM image (a) and TEM morphology (b) of prepared NiZnCo ferrite
The images of AEM and TFM for the NiZnCo ferrite (Fig.3) reveal the presence of a large quantity of nanoparticles with typical diameter of about 25 nm. During the homogeneous precipitation, the precipitating agent is slowly formed in the reaction mixture. The slow reaction course results in good crystallinity as well as regular shape and size of particles.
It has been shown in Fig.4 that the real part ε′ of relatively complex permittivity of the zinc ferrite is about 7. The value increases to 11 for sample in which the zinc ferrite is doped with the Ni2+ and Co2+ ion. In NiZnCo ferrite, divalent metal ion occupies tetrahedral site (Zn2+) or octahedral site (Co2+ and Ni2+), and trivalent iron ions are expected to occupy tetrahedral and octahedral site. The zinc ferrite doped with Ni2+ or Co2+ increases electron exchange between Fe3+ and Fe2+; and holes transfer between Co3+-Co2+ and Ni3+ and Ni2+ at octahedral site, which leads to increased polarization and dielectric constant. The electron exchange between Fe2+ and Fe3+ in n-type and the hole exchange between Ni3+ and Ni2+ in p-type ferrites result in local displacements of electrons or holes in the direction of the electric field, causing polarization[15]. The high frequency dielectric constants are mainly contributed by the electronic polarizations[16] and hence are almost independent of frequency. The dielectric constants ε′ of these samples are far lower than originally reported as 103 for NiZn ferrite from the conventional solid-state process[17]. However, the imaginary part of complex permittivity is below 1 except for 1.3 of sample doped with Ni2+ and Co2+ ions. We suggest the CTAB-assisted hydrothermal crystallite process can decrease the possibility of electron polarization among the grains, which results in the low values of the dielectric constant as well as the dielectric loss![]() Through the incorporation of the magnetic fillers, the low values of the permittivity of composites can be altered to achieve the maximal absorption of the electromagnetic energy in megahertz range.
Through the incorporation of the magnetic fillers, the low values of the permittivity of composites can be altered to achieve the maximal absorption of the electromagnetic energy in megahertz range.
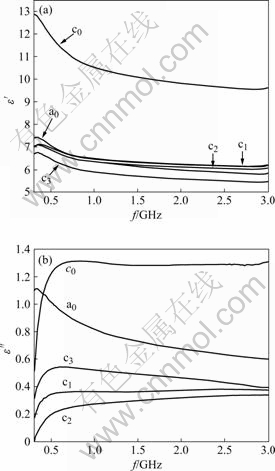
Fig.4 Complex permittivity of NiZn(Co) ferrite from CTAB-assisted hydrothermal process: (a) Real part; (b) Imaginary part
Fig.5 shows the frequency dependence on real μ′ and imaginary μ″ of relative complex permeability. The μ′′ of zinc ferrite reaches 1.5 at 300 MHz and drops to 1.2 at 1 GHz, while the μ′ of NiZnCo ferrite is obviously higher than 1.5. The μ′′ of NiZnCo ferrite reaches 5 at 300 MHz and drops to 3 at 1 GHz, which is better than that of the bulk NiZn ferrite. The μ″ of 1.5 for NiZnCo ferrite is larger than 0.2 for zinc ferrite at 1.25 GHz. This is concerned with the increased magnetic composition as well as the grain size of as-prepared ferrite. It can be assumed that some iron ions on octahedral sites may be in the divalent state via the hydrothermal process, which also affects the magnetic properties. The effect of cobalt ion on permeability is due to the distortion of the octahedral site[18]. These ferrites show relaxation resonance with decreasing μ′ drastically in the tested frequency range. The spin rotation plays a relatively more important role[12].
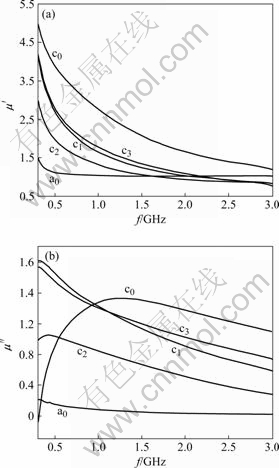
Fig.5 Complex permeability of NiZn(Co) ferrite via CTAB-assisted hydrothermal process: (a) Real part; (b) Imaginary part
In the case of single layered shielding material, the normalized input impedance Zin is given by Eq.(1):
![]() (1)
(1)
where Z0 is the wave impedance in free space; λ is the wavelength of the normal incident plane wave in free
space; t is the sample thickness; εr and μr are the relative complex permittivity and relative complex permeability, respectively. The reflection loss, RL(dB), can be obtained by
![]() (2)
(2)
Therefore, the attenuating reflection characteristics depend on the wavelength, layer thickness, relative complex permittivity and relative complex permeability. As the μ″ of as-prepared NiZnCo ferrite has values higher than 1.2 in the frequencies ranging from 0.7 to 3 GHz, the ferrite materials have high application for microwave absorbing fields in megahertz range.
From Fig.6, Ms of sample c0 is 0.074 A·m2/g and 0.041 and 0.057 A·m2/g for c2 and c3, respectively. The lowest Ms of sample c1 is 0.034 A·m2/g. The observed magnetization is a result of the simultaneous influence of several extrinsic and intrinsic factors such as anisotropy, grain size and the A–B exchange interaction. The change in Ms may be attributed to the change in Ni and Zn concentrations in spinel lattice. As the concentration of Fe3+ ions in sublattice is diluted by low concentrations of diamagnetic substitutions (Zn2+), the net magnetization increases[19]. However, magnetization decreases at higher levels of doping zinc from 0.40 to 0.63 mol. This can be attributed to the increased migration of Fe3+ ions from A- to B-sites with increasing Zn2+ content. This migration results in an increase in Fe3+ concentration on B-sites, which gives rise to anti-parallel spin coupling and spin canting[20], resulting in weakening of the A–B exchange interaction, reducing thereby the magnetization. In addition, Co2+ holds strong magnetic anisotropy Κ1 and magnetostrictive coefficient λs, and increases coercive force of spinel ferrite[11]. In fact, μ varies with Ms/Ha, where Ha is proportional to the magnetization component perpendicular to the high frequency field as well as magnetostriction coefficient.
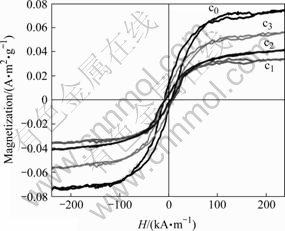
Fig.6 M-H loops for NiZn(Co) ferrite via CTAB-assisted hydrothermal process
4 Conclusions
1) Nanosized spinel NiZnCo ferrite was successfully prepared by CTAB-assisted hydro thermal method at 160 ℃.
2) The real part ε′ of complex permittivity of NiZnCo ferrite is about 11 and its imaginary part![]() is 1.3. The low dielectric constant behavior is quite useful for the high frequency application. The μ″ of NiZnCo ferrite has values higher than 1.2 within 0.7-3.0 GHz, so the ferrite materials can be used as microwave absorbing materials in megahertz frequency range.
is 1.3. The low dielectric constant behavior is quite useful for the high frequency application. The μ″ of NiZnCo ferrite has values higher than 1.2 within 0.7-3.0 GHz, so the ferrite materials can be used as microwave absorbing materials in megahertz frequency range.
3) With the small amount of Co into NiZn ferrite, the Ms of ferrite increases to 0.074 A·m2/g and the μ′ of NiZnCo ferrite reaches 5 at 300 MHz. This is related to magnetization component perpendicular to the high frequency field as well as magnetostriction coefficient.
References
[1] CRUICKSHANK D. 1-2 GHz dielectrics and ferrites: Overview and perspectives [J]. J Euro Ceram Soc, 2003, 23(14): 2721-2726.
[2] DAMNJANOVIC M, STOJANOVIC G, DESNICA V. Analysis, design, and characterization of ferrite EMI suppressors [J]. IEEE Trans Magn, 2006, 42(2): 270-277.
[3] ABDUN A M. Dielectric behaviour in Ni-Zn ferrites [J]. J Magn Magn Mater, 1999, 192(1): 121-129.
[4] GRIMAL V, AUTISSIER D, LONGUET L. Iron, nickel and zinc stoichiometric influences on the dynamic magneto-elastic properties of spinel ferrites [J]. J Euro Ceram Soc, 2006, 26(16): 3687-3693.
[5] VERMA A, GOEL T C, MENDIRATTA R G. Dielectric properties of NiZn ferrites prepared by the citrate precursor method [J]. Mater Sci Eng B, 1999, 60(2): 156-162.
[6] CALTUN O F, SPINU L. Magnetic properties of high frequency Ni-Zn ferrites doped with CuO [J]. IEEE Trans Magn, 2001, 37(4): 3353-3355.
[7] PARVATHEESWARA RAO B, CALTUN O F. Microstructure and magnetic behaviour of Ni-Zn-Co ferrites [J]. J Optoelectron Adv Mater, 2006, 8(3): 995-997.
[8] KOMARNENI S, FREGEAU E, BREVAL E. Hydrothermal preparation of ultrafine ferrites and their sintering [J]. J Am Ceram Soc, 1998, 71(1): 26-28.
[9] BURUKHIN A A, CHURAGULOV B R, OLEINIKOV N N. Synthesis of nanosized ferrite powders from hydrothermal and supercritical solutions [J]. Russian J Inorg Chem, 2001, 46(5): 646-651.
[10] ROZMAN M, DROFENIK M. Hydrothermal synthesis of manganese zinc ferrites [J]. J Am Chem Soc, 1995, 78(9): 2449-2455.
[11] O’HANDLEY R C. Modern magnetic materials principles and application [M]. ZHOU Yong-qia. Beijing: Chemical Industry Publishing House, 1996: 175-194, 214-231. (in Chinese)
[12] SHEN X, GONG R Z, FENG Z K. Effective permeability of NiZnCo ferrite granular thin films [J]. J Am Ceram Soc, 2007, 90(7): 2196-2199.
[13] DOSOUDIL R, OLAH V. Measurement of complex permeability in the RF band [J]. J Electric Eng, 2004, 55: 97-100.
[14] ROSEN M J. Surfactants and interfacial phenomena [M]. 3rd ed. New Jersey: J Wiley, 2004:105-108.
[15] AZADMANJIRI J, SALEHANI H K, BARATI M R. Preparation and electromagnetic properties of Ni1-xCuxFe2O4 nanoparticle ferrites by sol–gel auto-combustion method [J]. Mater Lett, 2007, 61: 84-87.
[16] HENCH L L, WEST J K. Principles of electronic ceramics [M]. New York: J Wiley, 1990: 189.
[17] KOOPS C G. On the dispersion of resistivity and dielectric constant of some semi-conductors at audiofrequencies [J]. Phys Rev, 1951, 83: 121-124.
[18] PEREIRA S L, PFANNES H D, FILHO A A M. A comparative study of NiZn ferrites modified by the addition of cobalt [J]. Mater Resea, 1999, 2(3): 231-234.
[19] NAKAMURA T. Low-temperature sintering of Ni-Zn-Cu ferrite and its permeability spectra [J]. J Magn Magn Mater, 1997, 168: 285-291.
[20] COSTA A C F M, SILVA V J, CORNEJO D R, MORELLI M R, KIMINAMI R H G A, GAMA L. Magnetic and structural properties of NiFe2O4 ferrite nanopowder doped with Zn2+[J]. J Magn Magn Mater, 2008, 320(14): 370-372.
Foundation item: Project(40830748) supported by the National Natural Science Foundation of China
Corresponding author: SHEN Xiang; Tel: +86-27-67884283; E-mail: xiangshen@yahoo.cn
DOI: 10.1016/S1003-6326(09)60075-3


