文章编号:1004-0609(2012)09-2662-05
电解锰阳极渣还原浸出锰
牛莎莎,王志兴,郭华军,李新海,彭文杰,胡启阳,张云河
(中南大学 冶金科学与工程学院,长沙 410083)
摘 要:
针对电解锰阳极渣难处理、铅含量高的缺点,提出利用桔子皮作还原剂在硫酸体系中还原浸出电解锰阳极渣工艺。以国内某电解锰厂阳极渣为原料,对桔子皮加入量、浸出时间、浸出温度以及硫酸加入量等工艺参数进行探讨和优化。结果表明:在浸出温度为80 ℃,时间为2 h,固液比为1:4,桔子皮/锰阳极渣质量比为1:5,酸渣质量比为1.2:1的条件下,锰的浸出率可达96%,铅的浸出率仅为0.2%,有效地实现了铅锰分离。实验证明,在硫酸体系中利用桔子皮作还原剂浸出电解锰阳极渣的方法可行。
关键词:
中图分类号:TF111 文献标志码:A
Reductive leaching of manganese from manganese anode slag
NIU Sha-sha, WANG Zhi-xing, GUO Hua-jun, LI Xin-hai, PENG Wen-jie, HU Qi-yang, ZHANG Yun-he
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Orange peel was used as reductant for reductive leaching of manganese anode slag, which was featured by intractable treatment and lead-rich, and sulfuric acid was used as leaching solvent. By using manganese anode slag from electrolytic manganese plant as raw materials, the effects of dosage of orange peel and sulfuric acid, leaching time and temperature on the leaching ratio were studied. The results show that the leaching recovery of Mn reaches 96%, while the leaching recovery of Pb is only 0.2% under the conditions of leaching temperature 80 ℃, leaching time 2h, the ratio of solid to liquid 1:4, the mass ratio of orange peel to slag 1:5 and the mass ratio of acid to material 1.2:1. The experiment proves that extracting manganese from anode slag in sulfuric acid using the orange peel as reductant is highly effective.
Key words: manganese anode slag; orange peel; reductive leaching
基金项目:湖南省科技计划重大专项(2011FJ1005)
收稿日期:2011-09-06;修订日期:2012-01-09
通信作者:王志兴,教授,博士;电话:0731-88836633;E-mail: zxwang@mail.csu.edu.cn
随着我国钢铁和有色金属工业的迅速发展,对作为钢铁及有色金属工业的添加剂、脱氧剂和脱硫剂的锰及其合金的需求不断增长。与之相应,电解金属锰行业亦得到迅速发展[1-2]。
电解法的优点是对矿石要求较低(可使用贫矿),并可获得高纯度金属,因而得到广泛应用[3],但其缺点是在电解过程中产生大量废水、废气和废渣,如在阳极区有大量阳极渣产生。这种阳极渣是由电解液中部分Mn被氧化而成,含有MnO2、PbSO4等数十种化合物,锰含量(质量分数)为42%~ 50%,因其组成复杂,回收利用难度大,除少量用于电解锰厂氧化二价铁外,绝大部分目前只能被廉价销售或堆存[4-5]。目前,针对电解锰阳极渣的处理方法主要有还原焙烧后浸出[6-7]、直接还原酸浸[8]以及生物浸出[9-10]。WANG等[11]采用闪锌矿和 MnO2的双电池系统研究了常规发电浸出过程和微生物协同发电浸出过程。结果表明,微生物协同发电浸出的浸出率和发电量比常规发电浸出的显著提高[12]。还原焙烧后浸出因其能耗过大、流程过长以及会产生大量的废渣已逐渐被淘汰。生物浸出因其细菌的数量和活性较难控制难以实现工业化。直接还原酸浸在有效提取锰的同时,可缩短工艺流程,减少能耗,因此,选择合适且廉价的还原剂成为研究热点。
中国是世界上主要的柑桔生产国之一,种植面积和产量分别居世界第一和第三。桔子皮作为柑桔加工的副产物,目前无法得到有效利用而废弃,不仅造成资源浪费,而且带来了环境污染问题[13]。桔子皮中富含纤维素、果胶等还原性成分,可作为还原剂。本文作者以某电解锰厂堆存的阳极渣为原料,选用廉价的植物还原剂桔子皮,采用湿法浸出工艺,提取阳极渣中的锰,同时达到锰铅分离的效果。
1 实验
1.1 实验原料
本实验采用某电解锰厂在生产过程中产生的电解锰阳极渣,含水量(质量分数)约为14.2%,阴离子主要是硫酸根离子。表1列出了电解锰阳极渣的主要化学成分,其中Mn含量很高,主要杂质为Pb,其他金属元素含量较少。阳极渣的平均粒径为11.14 μm,桔子皮的平均粒径为37.81 μm。实验所用水为去离子水。
表1 电解锰阳极渣主要化学成分分析
Table 1 Chemical composition of manganese anode slag (mass fraction, %)
![]()
1.2 实验原理
桔子皮-硫酸直接浸出阳极渣,反应属多相氧化还原反应,与其他如玉米秆、木屑等植物还原剂-酸浸反应机理大致相同[14]。桔子皮中含有纤维素、果胶、木质素等含碳有机物,MnO2在酸性条件下具有较强的氧化性,桔子皮中含碳有机物与MnO2发生氧化还原反应, MnO2被还原为Mn2+而进入溶液中[15]。主要反应如下:
C6H12O6 +12MnO2+24H+=12Mn2++6CO2+18H2O (1)
(C6H12O5)n+13nMnO2+26nH+ =13nMn2++6nCO2+19nH2O (2)
1.3 实验方法
称取阳极渣10 g,加入一定量的桔子皮,置于三口烧瓶中,阳极渣按1:4的固液比加入一定浓度的硫酸溶液,启动搅拌装置,在反应过程中保持固液比不变,在一定温度下水浴反应一定时间,抽滤料浆,滤渣烘干称量,然后利用原子吸收法测定溶液中锰和铅的含量,对滤渣中的剩余成分进行检测。
2 结果与讨论
2.1 原料物相分析
对电解锰阳极渣和桔子皮分别进行了XRD分析和红外光谱分析,分析结果如图1和2所示。

图1 电解锰阳极渣的XRD谱
Fig. 1 XRD pattern of manganese anode slag
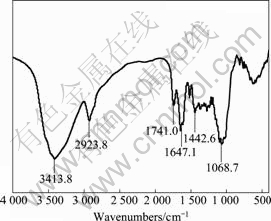
图2 桔子皮的红外光谱
Fig. 2 Infrared spectrum of orange peel
图1所示为阳极渣的XRD谱,阳极渣中物相复杂,主要为二氧化锰(MnO2)、铅锰氧化物(Pb2Mn8O16)、硫酸铅(PbSO4)等。
图2所示为桔子皮的红外光谱分析曲线。由文 献[16]可知,根据各自的红外波数的不同,将桔子皮的红外光谱分成不同组。在3 600~3 300 cm-1处宽而尖的吸收峰被认为是O—H官能团的伸缩作用。O—H在一个很大的频率范围内的伸缩振动表明存在“自由”形式的O—H和羧酸中的O—H。在2 926和2 923 cm-1处的吸收峰表明存在脂肪酸中的对称和非对称形式的C—H键的伸缩振动。
在1 750~1 600 cm-1处的吸收峰表明存在由于非离子形式的羧基类官能团(COOH,COOCH3)而产生的C=O的伸缩振动,这些非离子形式的羧酸类官能团可能为羧酸或者脂类。非对称的和对称的离子形式的羧酸类官能团(COO)的伸缩摇摆出现的吸收峰为 1 275 cm-1,而在1 372 cm-1处出现的吸收峰应该是果胶中的对称形式的COO的伸缩振动。在1 200~1 000 cm-1处的吸收峰可以认为是醇类和羧酸中的C—OH的伸缩振动。
2.2 桔子皮/锰阳极渣质量比对锰、铅浸出率的影响
图3所示为桔子皮/锰阳极渣质量比对锰、铅浸出率的影响。从图3可以看出,桔子皮加入量对锰的浸出率影响很大,随着桔子皮加入量的增加,锰的浸出率随之提高。在80 ℃下,当桔子皮/阳极渣质量比为1:5时,锰的浸出率可达96%以上。而随着桔子皮加入量的增加,铅的浸出率也有所提高,但其浸出率变化不大,最高仅有0.49%。
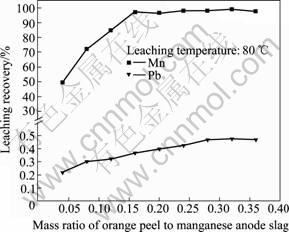
图3 桔子皮/锰阳极渣质量比对锰、铅浸出率的影响
Fig. 3 Effect of mass ratio of orange peel to manganese anode slag on leaching recoveries of manganese and lead
电解锰阳极渣中锰主要以二氧化锰形态存在,其次为铅锰化合物,其价态均高于+2价,在还原浸出过程中需要还原剂的参与,所以锰的浸出率随还原剂添加量的增加而增大。当桔子皮/阳极渣质量比达到1:5时,此时还原剂已足量,再增加桔子皮的量对锰浸出率无明显影响。反应体系为硫酸体系,SO42-浓度较高,Pb2+主要以PbSO4沉淀形式存在,因而Pb浸出率很低。
2.3 浸出温度对锰、铅浸出率的影响
图4所示为浸出温度对锰、铅浸出率的影响。从图4可以看出,浸出温度对锰的浸出率影响显著,随着浸出温度的升高,锰的浸出率随之显著提高。这是因为桔子皮的热解程度随温度的升高而增强,因此,在一定的浸出时间内,硫酸与桔子皮以及阳极渣中的锰、铅等之间的化学反应进行得更彻底;当温度升至80 ℃后,锰的浸出率随温度升高变化不大。当温度由30 ℃升至90 ℃时,Pb浸出率由0.34%提高到0.38%,这主要是PbSO4的溶解度随温度升高而提高所致。
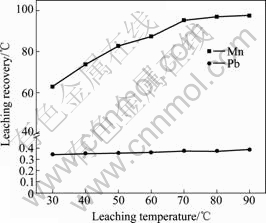
图4 浸出温度对锰、铅浸出率的影响
Fig. 4 Effect of leaching temperature on leaching recoveries of manganese and lead
2.4 浸出时间对锰浸出率的影响
图5所示为浸出时间对锰浸出率的影响。由图5可知,增加浸出时间可有效提高锰的浸出率,同时提高浸出温度可有效提高锰的浸出率。
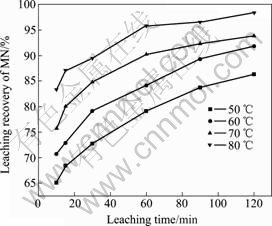
图5 浸出时间对锰浸出率的影响
Fig. 5 Effect of leaching time on leaching recovery of manganese
随着反应的进行,二氧化锰等化合物溶解进入溶液,PbSO4等不溶性杂质与反应核脱落悬浮在溶液中,反应界面不断收缩,严格来说,这一反应体系处于非稳定状态。反应过程中,通过搅拌加强了液相反应物及生产物的扩散,但还原剂的消耗使其本体浓度不断降低,因此,随着反应的进行,浸出反应速率不断降低,浸出率随时间的变化曲线趋于平缓。
2.5 酸渣质量比对锰、铅浸出率的影响
图6所示为酸渣质量比对锰、铅浸出率的影响。由图6可知锰的浸出率随着硫酸用量的增加而提高,当桔子皮/阳极渣质量比为1:5,酸渣质量比为1.2:1时,锰浸出率超过94%,之后继续增大酸渣质量比,锰的浸出率变化很小,在整个过程中,铅的浸出率稍有下降。原渣中锰铅质量比为8.2:1,分离之后溶液中锰铅质量比可达2250:1。
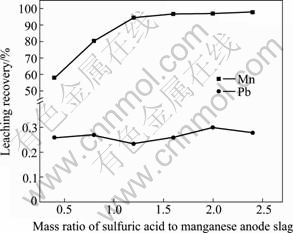
图6 酸渣质量比对铅、锰浸出率的影响(桔子皮添加量为2.0 g)
Fig. 6 Effect of mass ratio of sulfuric acid to manganese anode slag on leaching recoveries of manganese and lead (Orange peel dosage is 2.0 g)
由反应(1)和(2)可知,还原浸出反应为耗酸反应,在保持液固比恒定时,增大酸渣比可提高溶液中H+浓度,有利于反应正向进行,因而锰浸出率提高。另一方面,SO42-浓度亦随酸渣比增大而增大,同离子效应致使Pb浸出率降低,同时,SO42-浓度与Pb2+浓度存在数量级差距,所以Pb浸出率变化不明显。
3 结论
1) 利用桔子皮作还原剂,在硫酸溶液中浸出锰阳极渣,可有效地实现锰铅分离,96%的锰进入溶液中,而铅仅少量进入溶液,几乎全部留在沉淀中,锰铅分离效果显著。
2) 浸出最佳实验条件如下:浸出温度为80 ℃,桔子皮/锰阳极渣质量比为1:5,浸出时间为2 h,酸渣质量比为1.2:1,固液比为1:4。
REFERENCES
[1] 谭柱中. 中国电解金属锰工业现状[J]. 中国锰业, 2005, 23(2): 5-6.
TAN Zhu-zhong. EMM industry in China [J]. China’s Manganese Industry, 2005, 23(2): 5-6.
[2] 谭柱中, 梅光贵. 锰冶金学[M]. 长沙: 中南大学出版社, 2004.
TAN Zhu-zhong, MEI Guang-gui. Manganese metallurgy [M]. Changsha: Central South University Press, 2004.
[3] ZHANG Wen-sheng, CHENG Chu-yong. Manganese metallurgy review (Part Ⅰ): Leaching of ores/secondary materials and recovery of electrolytic/chemical manganese dioxide [J]. Hydrometallurgy, 2007, 89: 137-159.
[4] 梅光贵, 钟竹前. 湿法冶金新工艺[M]. 长沙: 中南大学出版社, 1994.
MEI Guang-gui, ZHONG Zu-qian. New technology of hydrometallurgy [M]. Changsha: Central South University Press, 1994.
[5] 申永强, 符智荣, 黄养逢, 石爱华, 颜文斌. 电解金属锰阳极泥回收制备化学二氧化锰工艺研究[J]. 中国锰业, 2007, 25(3): 14-16.
SHEN Yong-qiang, FU Zhi-rong, HUANG Yang-feng, SHI Ai-hua, YAN Wen-bin. The research on manganese anode slime recycle to produce into chemical manganese dioxide [J]. China’s Manganese Industry, 2007, 25(3): 14-16.
[6] ZHU Yang-ge, ZHANG Guo-fan, FENG Qi-ming, LU Yi-ping, OU Le-ming, HUANG Si-jie. Acid leaching of vanadium from roasted residue of stone coal [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(1): 107-111.
[7] CHENG Zhuo, ZHU Guo-cai, ZHAO Yu-na. Study in reduction-roast leaching manganese from low-grade manganese dioxide ores using cornstalk as reductant [J]. Hydrometallurgy, 2009, 96: 176-179.
[8] HARIPRASAD D, DASH B, GHOSH M K, ANAND S. Leaching of manganese ores using sawdust as a reductant [J]. Minerals Engineering, 2007, 20: 1293-1295.
[9] ACHARYA C, KAR R N, SUKLA L B. Studies on reaction mechanism of bioleaching of manganese ore [J]. Minerals Engineering, 2003, 16(10): 1027-1030.
[10] XIN Bao-ping, CHEN Bing, DUANB Ning, ZHOU Chang-bo. Extraction of manganese from electrolytic manganese residue by bioleaching [J]. Bioresource Technology, 2011, 102: 1683-1687.
[11] WANG Shao-fen, XIAO Li, FANG Zheng, QIU Guan-zhou, WANG Chun-xiong. Electrogenerative leaching for sphalerite- MnO2 in the presence of Acidithiobacillus thiooxidans [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(1): 21-25.
[12] WANG Shao-fen, XIAO Li, FANG Zheng, QIU Guan-zhou, WANG Chun-xiong. Mechanism of electro-generative-leaching of chalcopyrite-MnO2 in presence of Acidithiobacillus ferrooxidans [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(1): 15-20.
[13] 乔海鸥, 丁晓雯, 张庆祝. 柑橘皮的综合利用[J]. 浙江农业科学, 2003(3): 147-149.
QIAO Hai-ou, DING Xiao-wen, ZHANG Qing-zhu. Research on the extracting condition of useful substances from the orange peels [J]. Journal of Agricultural Science, 2003(3): 147-149.
[14] TRIFONI M, VEGLIO F, TAGLIERI G, TORO L. Acid leaching process by using glucose as reducing agent: A comparison among the efficiency of different kinds of manganiferous ores [J]. Minerals Engineering, 2000, 13(2): 217-221.
[15] TIAN Xi-ke, WEN Xiao-xia, YANG Chao, LIANG Yu-jun, PI Zheng-bang, WANG Yan-xin. Reductive leaching of manganese from low-grade manganese dioxide ores using corncob as reductant in sulfuric acid solution [J]. Hydrometallurgy, 2010, 100: 157-160.
[16] IQBAL M, SAEED A, IQBAL Z S. FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste [J]. Journal of Hazardous Materials, 2009, 164(l): 161-171.
摘 要:针对电解锰阳极渣难处理、铅含量高的缺点,提出利用桔子皮作还原剂在硫酸体系中还原浸出电解锰阳极渣工艺。以国内某电解锰厂阳极渣为原料,对桔子皮加入量、浸出时间、浸出温度以及硫酸加入量等工艺参数进行探讨和优化。结果表明:在浸出温度为80 ℃,时间为2 h,固液比为1:4,桔子皮/锰阳极渣质量比为1:5,酸渣质量比为1.2:1的条件下,锰的浸出率可达96%,铅的浸出率仅为0.2%,有效地实现了铅锰分离。实验证明,在硫酸体系中利用桔子皮作还原剂浸出电解锰阳极渣的方法可行。


