
Mechanism of mechanical activation for sulfide ores
HU Hui-ping(胡慧萍)1, 2, CHEN Qi-yuan(陈启元)1, YIN Zhou-lan(尹周澜)1,
HE Yue-hui(贺跃辉)2, HUANG Bai-yun(黄伯云)2
1. Institute of Metallurgical and Applied Physico-chemistry, School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. State Key Laboratory of Powder Metallurgy, Powder Metallurgy Research Institute, Central South University, Changsha 410083, China
Received 14 May 2006; accepted 12 November 2006
Abstract:
Structural changes for mechanically activated pyrite, sphalerite, galena and molybdenite with or without the exposure to ambient air, were systematically investigated using X-ray diffraction analysis(XRD), particle size analysis, gravimetrical method, X-ray photo-electron spectroscopy(XPS) and scanning electron microscopy(SEM), respectively. Based on the above structural changes for mechanically activated sulfide ores and related reports by other researchers, several qualitative rules of the mechanisms and the effects of mechanical activation for sulfide ores are obtained. For brittle sulfide ores with thermal instability, and incomplete cleavage plane or extremely incomplete cleavage plane, the mechanism of mechanical activation is that a great amount of surface reactive sites are formed during their mechanical activation. The effects of mechanical activation are apparent. For brittle sulfide ores with thermal instability, and complete cleavage plane, the mechanism of mechanical activation is that a great amount of surface reactive sites are formed, and lattice deformation happens during their mechanical activation. The effects of mechanical activation are apparent. For brittle sulfide ores with excellent thermal stability, and complete cleavage plane, the mechanism of mechanical activation is that lattice deformation happens during their mechanical activation. The effects of mechanical activation are apparent. For sulfide ores with high toughness, good thermal stability and very excellent complete cleavage plane, the mechanism of mechanical activation is that lattice deformation happens during their mechanical activation, but the lattice deformation ratio is very small. The effects of mechanical activation are worst.
Key words:
sulfide ore; mechanism; mechanical activation; structural change;
1 Introduction
The use of mechanical activation as a means of accelerating leaching is not new. GERLACH and GOCK [1] granted a German patent in 1973, which included the application of mechanical activation to sulphide ores. BALAZ and EBERT[2] referenced five studies, dated from 1977 to 1987, which demonstrated this effect on the leaching of zinc from sphalerite. MAURICE and HAWK[3] investigated ferric chloride leaching of mechanically activated chalcopyrite. Ultrafine grinding of chalcopyrite increases its reactivity so that less severe leaching conditions are required to recover the copper[4-5]. In the past 10 years, much focused effort has been made to quantify the enhancement due to mechanical activation[6-8]. Thus the term ‘mechanical activation’ refers to mechanically induced enhancement of the chemical reactivity of a system[9], which is attributed to increased specific surface area, enhanced surface reactivity, and changes in crystalline structure.
Whereas, according to our previous research work [10-11], mechanical activation can effectively intensify hydrometallurgical processes of predominant sulfide ores that are difficult to be leached, but can’t apparently enhance hydrometallurgical processes for some sulfide ores, such as molybdenite. Nowadays, most research works just describe experimental phenomena to single mineral, and no suitable theory can forecast the effect of mechanical activation. Therefore, it is necessary to find the general rules in order to predict the mechanisms and the effects of mechanical activation for different sulfide ores.
In this paper, structural changes for mechanically activated pyrite, sphalerite, galena and molybdenite were studied. Based on the structural changes for mechanically activated sulfide ores and related reports by other researchers, several qualitative rules of the mechanisms and the effects of mechanical activation for sulfide ores are obtained.
2 Experimental
2.1 Materials
Natural pyrite ore was purchased from a domestic geological museum. Naturally pure hand-sorted galena, sphalerite and molybdenite ores were purchased from a domestic mine, and their chemical compositions are presented in Tables 1-4. It was found by X-ray diffraction analysis that the natural pyrite, sphalerite and galena contained cubic pyrite, cubic sphalerite and cubic galena as a predominant component, respectively. And the natural molybdenite contained hexagonal molybdenite as a predominant component. The natural molybdenite was floated with about 5×10-5 xanthate as a floatation reagent. 40 g floated molybdenite and 200 mL CCl4 were added into a 250 mL flask, refluxed for 72 h, and then filtrated to leave xanthate in the filtrate and obtain a filter residue. The filter residue was dried at 80 ℃ in vacuum to yield unactivated molybdenite. The unactivated pyrite or sphalerite or galena was prepared by crushing respective ores in a jaw crusher to a certain particle size, then stored in air for more than 1 year, sieved to obtain above 1 mm pyrite or sphalerite or galena.
Table 1 Chemical composition of natural pyrite (mass fraction, %)
![]()
Table 2 Chemical composition of natural sphalerite (mass fraction, %)
![]()
Table 3 Chemical composition of natural galena (mass fraction, %)
![]()
Table 4 Chemical composition of natural molybdenite (mass fraction, %)
![]()
2.2 Mechanical activation
The unactivated sulfide ore (weighed charge in mill 10 g) was added into a stainless steel vessel with 6 stainless steel balls of 18 mm in diameter and 12 balls of 8 mm in diameter. A vacuum with residue pressure ≤1 Pa was then pulled on the vessel followed by gassing highly pure nitrogen (99.999%, volume fraction) (abbreviated as nitrogen) through an inlet of this vessel for 1.5 h (the ore was considered being kept under inert atmosphere). Or this vessel was filled with laboratory air, then two inlets of this vessel were sealed (the ore was kept under less air). Or two inlets of this vessel were open (the ore was exposed to air). And then the sulphide ores were mechanically activated in a planetary ball mill (QM-ISP Planetary mill, China) under a rotation speed of 200 r/min. A powder-to-ball mass ratio of 1?25 was employed. Mechanically activated sulfide ores were obtained after grinding for tG=20, 40, 120, 180, 260, 480 min, respectively.
2.3 Aging experiments
Pyrite, sphalerite and galena mechanically activated in nitrogen for 20 or 120 min were exposed to air for 3 months or 6 months, to obtain mechanically activated pyrite, sphalerite and galena aged in air, respectively.
2.4 Physico-chemical characteristics
X-ray powder diffraction analysis(XRD) for the milled powders was recorded on a 3014 diffractometer (Rigaku, Japan) using Cu Kα radiation (l=1.54 ?, voltage 40 kV, current 20 mA) with step size of 0.03?. The recorded XRD spectra were used for calculation of the degree of structural disorder. The lattice deformation ratio(ε) (presented as a percentage) and the crystallite size(D) were determined from the changes in profile of the diffraction peaks, such as (220) and (551) planes of pyrite, (111) and (331) planes of sphalerite, (200) and (400) planes of galena, (002) and (008) planes of molybdenite. The lattice deformation ratio(ε) and the crystallite size(D) can be calculated using Gaussian function[12].
X-ray photoelectron spectra were obtained with a KRATOS XSAM800 X-ray photoelectron spectrometer, with a monochromatized Mg Ka X-ray source. The operating pressure in the analyzer chamber was ≤2×10-7 Pa. The surface charge was measured relative to uncharged graphite C 1s at 284.6 eV.
The specific granulometric surface areas(SG) of mechanically activated sulfide ores were calculated statistically from the corresponding particle size distribution data measured on a Mastersizer 2000 Laser Diffraction Particle Size Analyzer (Malvern, Great Britain). For pyrite, sphalerite and galena, distilled water was used as a dispersing agent. For molybdenite, ethyl alcohol (95%, volume fraction) was used as a dispersing agent.
The elemental sulphur contents of unactivated and mechanically activated sulfide ores were determined by using gravimetric method. And a typical operation was performed as follows. 2.0 g mechanically activated pyrite and 20 mL CCl4 were added into a 50 mL flask, refluxed for 72 h, and finally filtered. CCl4 was then evaporated at ambient temperature to yield elemental sulphur, which was then weighed to determine the elemental sulphur content.
The morphology analysis of unactivated or mechanically activated sulfide ores was performed using JSM-5600LV Scanning Electron Microscopy (JEOL, Japan).
3 Results and discussion
3.1 Structural changes of sulfide ores mechanically activated under different grinding atmospheres
The specific surface areas (SG) of pyrite, sphalerite and galena mechanically activated under nitrogen increase with increasing grinding time, and remain constant after a certain grinding period[13-14]. This shows that the increase of SG isn’t a major factor for the enhancement of the hydrometallurgical process of mechanically activated sulphide ores. However, the specific surface areas (SG) of molybdenite mechanically activated under nitrogen increase with increasing grinding time(Fig.1), which indicates that the mechanism of mechanical activation of molybdenite is quite different from that of other sulfide ores.
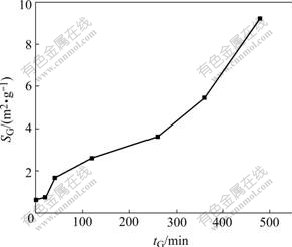
Fig.1 Specific granulometric surface area SG of mechanically activated molybdenites vs grinding time
XRD patterns of pyrites, galena and sphalerite mechanically activated under different atmospheres for 120 min show that new phases can’t be found[13-14].
S 2p XPS and Zn 2p XPS spectra of mechanically activated sphalerites, S 2p XPS and Fe 2p XPS spectra of mechanically activated pyrites, Pb 4f XPS and S 2p XPS spectra of mechanically activated galena are shown in Figs.2-7.
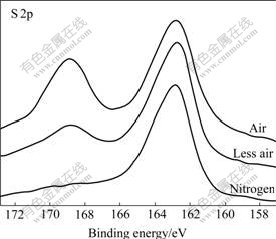
Fig.2 S 2p XPS spectra of pyrite mechanically activated under different grinding atmospheres for 120 min
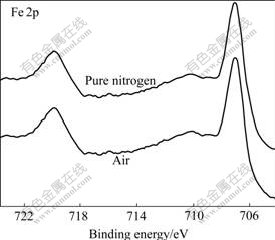
Fig.3 Fe 2p XPS spectra of pyrite mechanically activated under different grinding atmospheres for 120 min
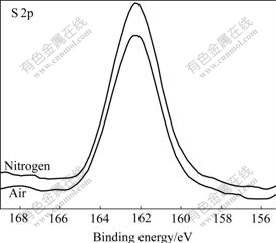
Fig.4 S 2p XPS spectra of sphalerite mechanically activated under different grinding atmospheres for 120 min
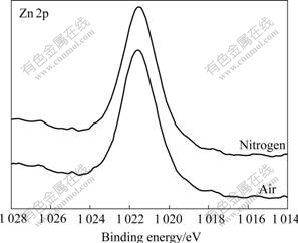
Fig.5 Zn 2p XPS spectra of sphalerite mechanically activated under different grinding atmospheres for 120 min
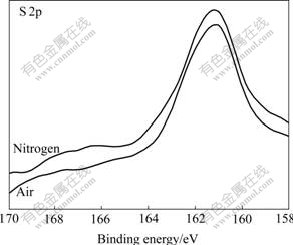
Fig.6 S 2p XPS spectra of galena mechanically activated under different grinding atmospheres for 120 min
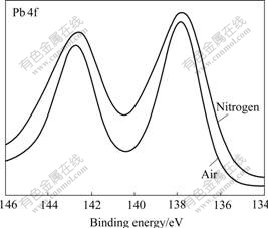
Fig.7 Pb 4f XPS spectra of galena mechanically activated under different grinding atmospheres for 120min
Figs.2-7 show that S 2p and Zn 2p XPS spectra of mechanically activated sphalerite, Fe 2p XPS spectra of mechanically activated pyrite, Pb 4f XPS and S 2p XPS spectra of mechanically activated galena don’t change, while the S 2p XPS spectra of mechanically activated pyrites are changed with grinding atmosphere. The significant differences in both shape and binding energy of the S 2p XPS spectra of pyrite (Fig.2) show that one new S 2p peak occurs in the range from 168 eV to 170 eV for pyrite mechanically activated under air or less air. The corresponding S 2p binding energies at 163.2 eV and 169.3 eV are for pyrite ![]() species[15] and S6+ species of
species[15] and S6+ species of ![]() [16], respectively, which shows that the relative content of the newly formed FeSO4 for mechanically activated pyrite increases in a sequence of grinding atmosphere of pure nitrogen, less air and air. EYMERY and YLLI[17] investigated a structural transformation in pyrite mechanically activated under air for 3 h and 60 h using X-ray diffraction analysis(XRD) and 57Fe M?ssbauer spectroscopy and a newly formed FeSO4 was also found. TAKAJI et al[18] studied the excellent complete cleavage plane (0001) of molybdenite using scanning Auger microscope, and the result showed that the excellent complete cleavage plane (0001) of molybdenite cannot absorb oxygen, which is the reason why molybdenite can be a lubricant for high vacuum pump[19].
[16], respectively, which shows that the relative content of the newly formed FeSO4 for mechanically activated pyrite increases in a sequence of grinding atmosphere of pure nitrogen, less air and air. EYMERY and YLLI[17] investigated a structural transformation in pyrite mechanically activated under air for 3 h and 60 h using X-ray diffraction analysis(XRD) and 57Fe M?ssbauer spectroscopy and a newly formed FeSO4 was also found. TAKAJI et al[18] studied the excellent complete cleavage plane (0001) of molybdenite using scanning Auger microscope, and the result showed that the excellent complete cleavage plane (0001) of molybdenite cannot absorb oxygen, which is the reason why molybdenite can be a lubricant for high vacuum pump[19].
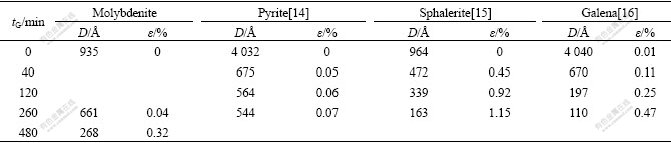
The lattice deformation ratio(ε) and the crystallite size(D) of mechanically activated sulfide ores are listed in Table 5. Table 5 shows that lattice deformation ratio increases with increasing grinding time, and the lattice deformation ratios(ε) of mechanically activated pyrite, sphalerite, galena and molybdenite are 0.07%, 1.15%, 0.47% and 0.04% at tG=260 min, respectively.
Table 5 Relationship between D, ε of different sulphide ores and grinding time tG
Almost no elemental sulfur is produced when sphalerite and molybdenite are mechanically activated under nitrogen. The contents of the elemental sulphur for pyrite and galena mechanically activated under nitrogen are listed in Tables 6 and 7, respectively. The contents of the elemental sulphur for mechanically activated pyrite are larger than those for mechanically activated galena. The formation of elemental sulfur during mechanical activation results in the formation of reactive sites on the surface of mechanically activated sulfide ores. Therefore, it’s easier for pyrite to form reactive sites than galena during mechanical activation, but it’s quite difficult for sphalerite and molybdenite to form reactive sites during mechanical activation.
Table 6 Elemental sulphur content of pyrite versus grinding time
![]()
Table 7 Elemental sulphur content of galena versus grinding time
![]()
The morphologies of non-activated and mechanically activated molybdenite, pyrite, sphalerite and galena are shown in Figs.8-11. As shown in Figs.8-11, pyrite, sphalerite and galena are broken into rough particles or rods by grinding for 120 min, but the layered structure of molybdenite is kept after grinding for 120 min. Because pyrite has a plane of incomplete cleavage, sphalerite and galena have planes of complete cleavage[20], it is easier for these sulphide ores to break into small particles or rods. Whereas, molybdenite belongs to a family of layered transition metal dichalcogenides with closedly packed planes of transition metal atoms(M) sandwiched between two similar planes of chalcogen atoms(X) and has very excellent complete cleavage. These X-M-X slabs are weakly bonded by van der Waals forces, while bonding within the X-M-X slabs is mainly covalent and fairly strong[21]. When molybdenite is intensively ground, it is easy for molybdenite to slip along the plane of very excellent cleavage.

Fig.8 SEM images for non-activated pyrite (a) and pyrite mechanically activated for 120 min under nitrogen (b)
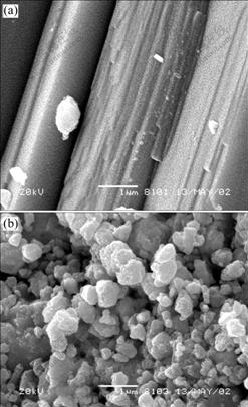
Fig.9 SEM images for non-activated galena (a) and galena mechanically activated for 120 min under nitrogen (b)

Fig.10 SEM images for non-activated sphalerite (a) and sphalerite mechanically activated for 120 min under nitrogen (b)
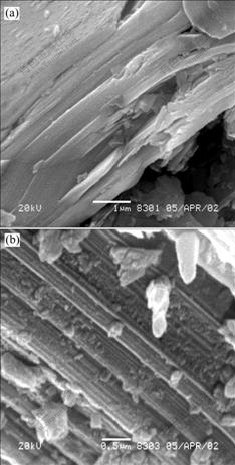
Fig.11 SEM images for non-activated molybdenite (a) and molybdenite mechanically activated for 120 min under nitrogen (b)
3.2 Structural changes of mechanically activated sulfide ores after exposure to ambient air
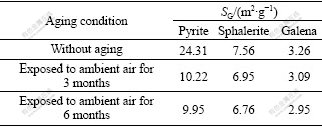
The specific granulometric surface areas(SG) of pyrite, sphalerite and galena mechanically activated in nitrogen for 120 min, followed by exposure to ambient air, are listed in Table 8.
Table 8 Relationship of SG of sulfide ores mechanically activated under nitrogen for 120 min with aging conditions
XRD patterns of pyrites, sphalerite and galena mechanically activated in nitrogen for 20 min or 120 min, followed by exposure to ambient air are shown in Figs.12-14.
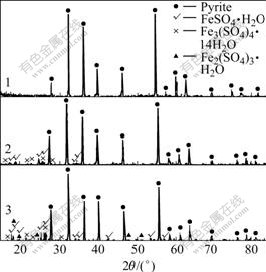
Fig.12 XRD patterns of pyrite mechanically activated in nitrogen for 20 min, followed by exposure to air at ambient temperature for a certain period: 1 Without aging; 2 Exposed to air for 3 months; 3 Exposed to air for 6 months
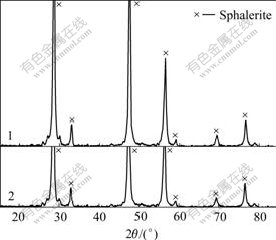
Fig.13 XRD patterns of sphalerite mechanically activated in nitrogen for 120 min, followed by aging under different conditions: 1 Without aging; 2 Exposed to ambient air for 6 months
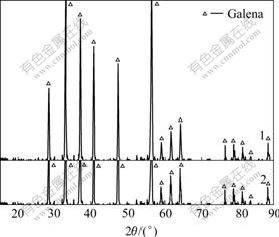
Fig.14 XRD patterns of galena mechanically activated in nitrogen for 120 min, followed by aging under different conditions: 1 Without aging; 2 Exposed to ambient air for 6 months
XPS spectra of mechanically activated pyrite and galena after exposure to ambient air for different periods are shown in Figs.15 and 16.
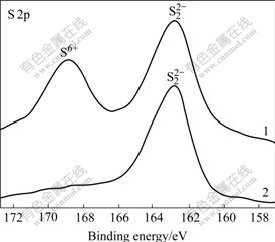
Fig.15 S 2p XPS spectra of pyrite mechanically activated in nitrogen for 20 min, followed by aging under different conditions: 1 Exposed to ambient air for 6 months; 2 Without aging
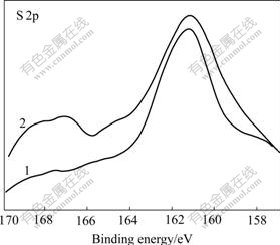
Fig.16 S 2p XPS spectra of galena mechanically activated in nitrogen for 20 min, followed by aging under different conditions: 1 Without aging; 2 Exposed to ambient air for 6 months
Although no oxidation phenomena are observed during mechanical activation of galena in air and after being aged of mechanically activated sphalerite for 6 months in ambient air, mechanically activated pyrite and galena were partially oxidized after being aged in ambient air for 6 months. The sensitivity to ambient aging air increases in the order of sphalerite, galena and pyrite. Therefore, the sensitivity to grinding atmosphere during mechanical activation of sulfide ores increases in the order of sphalerite (and molybdenite), galena and pyrite.
Why is it easier for galena and pyrite to produce elemental sulfur or to absorb oxygen than other sulfide ores during mechanical activation? Why are there differences of lattice deformation ratio among different sulfide ores during mechanical activation? Why are the lattice deformation ratio of mechanically activated pyrite and molybdenite too small? According to our previous research work [10-11], mechanical activation can apparently reinforce the hydrometallurgical process of pyrite, galena and sphalerite, but cannot apparently reinforce the hydrometallurgical process of molybdenite. In order to answer these questions, some explanations will be given below.
Physico-chemical properties of some common sulfide ores[20] are listed in Table 9. Table 9 shows that pyrite has extremely incomplete (100) and (111) cleavages, sphalerite has a complete (110) cleavage, and galena has a complete (100) cleavage. Pyrite has much more bond linkages between two cleavage planes than sphalerite or galena. For molybdenite, no chemical bond linkages exist between two very excellent complete cleavage planes. When these sulfide ores are intensively ground, the amount of reactive sites produced under the same mechanical activation condition decreases in the order of mechanically activated pyrite, mechanically activated sphalerite (and mechanically activated galena), and mechanically activated molybdenite.
On the other hand, the elemental sulfur is produced during mechanical activation of pyrite and galena. No elemental sulfur is produced during mechanical activation of sphalerite and molybdenite. Therefore, the overall amount of reactive sites produced under the same mechanical activation condition decreases in the order of mechanically activated pyrite, mechanically activated galena, mechanically activated sphalerite and mechanically activated molybdenite. The more the reactive sites there exist on the surface of mechanically activated sulfide ores, the more sensitive to oxidative grinding atmosphere or ambient aging air the mechanically activated sulfide ores are. That’s why it is easier for galena and pyrite to absorb oxygen than other sulfide ores during mechanical activation or exposure to ambient air.
Table 9 Physico-chemical properties of some common sulfide ores[20]
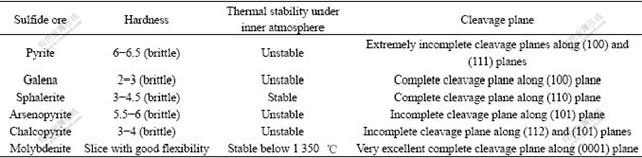
Table 9 shows that pyrite and galena are thermally unstable under inert atmosphere. For molybdenite, it’s thermally stable below 1 350℃ under inert atmosphere. For sphalerite, it’s thermally stable under inert atmosphere. Our research group also determined the initial thermal decomposition temperatures of sulfide ores under highly pure nitrogen using thermogravimetric method, and the initial thermal decomposition temperature of pyrite (713 K)[22] is lower than that of galena (1 140 K) [14]. No thermal decomposition pheno- mena were observed when sphalerite and molybdenite were heated to 1 100 ℃ under highly pure nitrogen[11]. According to the physical model about mechanical energy transformation reported by other researchers[23], when mechanical energy is transformed into chemical energy during mechanical activation of substances, chemical reaction happens on these “micro-contacting” points where the temperature reaches about 1 300 K.
This model can explain why no elemental sulfur is formed during mechanical activation of molybdenite and sphalerite, and more elemental sulfur is produced during mechanical activation of pyrite than that of galena.
There are experimental results reported by other researchers: 1) During mechanical activation of chalcopyrite under inert atmosphere, elemental sulfur was produced; sulfate was produced during grinding under air, which showed that chalcopyrite was sensitive to oxidative grinding atmosphere[24]. 2) During mechanical activation of arsenopyrite under inert atmosphere, a part of arsenopyrite was decomposed into ferrous sulfide and arsenic sulfide, and sulfate salts were produced during grinding under air, which showed that arsenopyrite was sensitive to oxidative grinding atmosphere[25-26]. 3) Lattice deformation ratios during mechanical activation of these sulfide ores that are thermally unstable were very small[10,27]. And the effects of mechanical activation on the hydrometallurgical behaviors for chalcopyrite and arsenopyrite are apparent[10,27]. From Table 9, chalcopyrite and arsenopyrite have incomplete cleavage plane and thermal instability under inert atmosphere.
Therefore, for brittle sulfide ore with thermal instability and incomplete cleavage plane, the main factor to reinforce its hydrometallurgical process is newly formed reactive sites during mechanical activation. And the effect of mechanical activation on its hydrometallurgical behavior is apparent.
For brittle materials with good thermal stability and complete cleavage plane, such as sphalerite, the smaller the hardness of brittle materials, the bigger the plastic deformation is when these brittle materials are put under external force interaction[28], and the bigger the lattice deformation ratio is.
For brittle materials with thermal instability and complete or incomplete cleavage plane, such as galena and pyrite, when these brittle materials are put under external force interaction, one part of mechanical energy is transformed into chemical energy (for example, thermal decomposition happens during external force interaction), and one part of mechanical energy will lead to lattice deformation of materials. Therefore, for these materials, the more chemical energy the mechanical energy is transformed, the less the lattice deformation ratio is.
For tough materials with very excellent complete cleavage plane and high thermal decomposition temperature under inert atmosphere, such as molybdenite, the cleavage plane is also the slip plane[28]. When molybdenite is intensively ground, it is easy for molybdenite to slip along the plane of very complete cleavage plane, which results in its less lattice deformation and its increase of surface area, but the lattice deformation ratio is very small.
Therefore, if sulfide ores have good thermal stability and very excellent complete or complete cleavage planes, the mechanism of mechanical activation for these ores is that lattice deformation happens during mechanical activation. And these sulfide ores are insensitive to oxidative grinding atmosphere. But the effect of mechanical activation on its hydrometallurgical behavior is not apparent.
4 Conclusions
1) For brittle sulfide ores with thermal instability, and incomplete cleavage plane or extremely incomplete cleavage plane, the mechanism of mechanical activation is that a great amount of surface reactive sites are formed during their mechanical activation. The effects of mechanical activation are apparent.
2) For brittle sulfide ores with thermal instability and complete cleavage plane, the mechanism of mechanical activation is that a great amount of surface reactive sites are formed, and lattice deformation happens during their mechanical activation. The effects of mechanical activation are apparent.
3) For brittle sulfide ores with excellent thermal stability and complete cleavage plane, the mechanism of mechanical activation is that lattice deformation happens during their mechanical activation. The effects of mechanical activation are apparent.
4) For sulfide ores with high toughness, good thermal stability and very excellent complete cleavage plane, the mechanism of mechanical activation is that lattice deformation happens during their mechanical activation, but the lattice deformation ratio is very small. The effects of mechanical activation are worst.
Acknowledgements We thank Mr. Kirch and Mr. Günter Gotlstein (Institute of Physical Metallurgy and Metal Physics, RWTH Aachen, Germany) for the SEM measurements. References
[1] GERLACH J K, GOCK E D. Leaching behavior of mechanically activated sphalerite [P]. Ger. Pat. 2,138,143 (NIM Tr. 430). 1973.
[2] BALAZ P, EBERT I. Oxidative leaching of mechanically activated sphalerite [J]. Hydrometallurgy, 1991, 27: 141-150.
[3] MAURICE D, HAWK J A. Ferric chloride leaching of mechanically activated chalcopyrite [J]. Hydrometallurgy, 1998, 49: 103-123.
[4] GOCK E. Beeinflussing des loseverhalterns von kupferkies durch festkorperreaktionen bei der schwingmahlung [J]. Erzmetall, 1978, 31: 282-288.
[5] CORRANS I J, ANGOVE J E. Ultrafine milling for the recovery of refractory gold [J]. Minerals Engineering, 1991, 4(7-11): 763-776.
[6] TKACOVA K, BALAZ P, MISURA B, VIGDERGAUZ V E, CHANTURIYA V A. Selective leaching of zinc from mechanically activated complex Cu-Pb-Zn concentrate [J]. Hydrometallurgy, 1993, 33: 291-300.
[7] BALAZ P, BRUANCIN J, SEPELAK V, HAVLIK T, SKROBIAN M. Non-oxidative leaching of mechanically activated stibnite [J]. Hydrometallurgy, 1992, 31: 201-212.
[8] TKACOVA K, BALAZ P. Structural and temperature sensitivity of leaching of chalcopyrite with iron(III) sulfate [J]. Hydrometallurgy, 1988, 21: 103-112.
[9] WARRIS C J, MCCORMICK P G. Mechanochemical processing of refractory pyrite [J]. Minerals Engineering, 1997, 10(10): 1119-1125.
[10] ZHAO Zhong-wei. Leaching Dynamics of Mechanically Activated Sulfide Ores [D]. Changsha: Central South University of Technology, 1999. (in Chinese)
[11] HU Hui-ping. Fundamental Research on Mechanically Activated Sulfide Ores [D]. Changsha: Central South University, 2003. (in Chinese)
[12] KLUG H P, ALEXANDER L. X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials (2ne ed.) [M]. New York: John Wiley & Sons, 1974: 618-708.
[13] HU Hui-ping, CHEN Qi-yuan, YIN Zou-lan. Effect of grinding atmosphere on the leaching of mechanically activated pyrites and sphalerites [J]. Hydrometallurgy, 2004, 72(1/2): 79-86.
[14] HU Hui-ping, CHEN Qi-yuan, YIN Zhou-lan. A study on thermal behaviors of mechanically activated galena [J]. Metallurgical and Materials Transactions A, 2003, 34A: 793-797.
[15] MYCROFT J R, BANCROFT G M, MCLNTYRE N S, LORIMER J W, HILL I R. Detection of sulphur and polysulphides on electrochemically oxidized pyrite surfaces by X-ray photoelectron spectroscopy and Raman spectroscopy [J]. J Electroanal Chem, 1990(292): 139-152.
[16] YELLOJIRAO M K, NATARAJAN K A. Effect of electrochemical interactions among sulphide minerals and grinding medium on the flotation of sphalerite and galena [J]. Int J Miner Process, 1990(29): 175-194.
[17] EYMERY J P, YLLI F. Study of a mechanochemical transformation in iron pyrite [J]. Journal of Alloys and Compounds, 2000, 298: 306-309.
[18] TAKAJI S, MURAKAMI K, GOTOH T. Observation of molybdenite surface using scanning auger microscope [J]. Applied Surface Science, 1999, 144/145: 278-282.
[19] ZHU M H, ZHOU Z R. An investigation of molybdenum disulfide bonded solid lubricant coatings in fretting conditions [J]. Surface and Coatings Technology, 2001, 141(2/3): 240-245.
[20] WANG Pu, PAN Zhao-lu, WEN Ling-bao. Systematic Mineralogy [M]. Beijing: Geology Press of China, 1982. (in Chinese)
[21] ALEXIEW V, PRINS R, WEBER T. Ab initio study of MoS2 and Li adsorbed on the ![]() face of MoS2 [J]. Phys Chem Chem Phys, 2000, 2(8): 1815-1827.
face of MoS2 [J]. Phys Chem Chem Phys, 2000, 2(8): 1815-1827.
[22] HU Hui-ping, CHEN Qi-yuan, YIN Zhou-lan, CHE Hong-sheng. Thermal behaviour of mechanically activated pyrites by thermogravimetry (TG) [J]. Thermochimica Acta, 2002, 398: 233-240.
[23] TKACOVA K. Mechanical Activation of Minerals [M]. Amsterdam: Elsevier, 1989: 35-37.
[24] DE DONATO P, KONGOLO M, BARRES O, YVON J. Chemical surface modifications of sulphide minerals after comminution [J]. Powder Technology, 1999, 105(1/3): 141-148.
[25] AYLMORE M G, LINCOLN F J. Mechanochemical milling-induced reactions between gases and sulfide minerals(II): Reactions of CO with arsenopyrite, pyrrhotite and pyrite [J]. Journal of Alloys and Compounds, 2001, 314: 103-113.
[26] WELHAM N J. Mechanochemical processing of gold-bearing sulphides [J]. Minerals Engineering, 2001, 14(3): 341-347.
[27] LI Hong-gui, YANG Jia-hong, ZHAO Zhong-wei, LI Yun-jiao. Leaching behavior of mechanically activated chalcopyrite [J]. Journal of Central South University of Technology, 1998, 29(1): 28-30.
[28] WANG Zi-qiang, DUAN Zhu-ping. Plastic Mechanics for Crystal Systematic Mineralogy [M]. Beijing: Science Press, 1995. (in Chinese)
Foundation item: Project(59934080) supported by the National Natural Science Foundation of China; Project(2000053321) supported by the Doctorate Program Fund of Ministry of Education, China
Corresponding author: HU Hui-ping; Tel: +86-731-8877364; E-mail: huhuiping69@vip.sina.com


