文章编号:1004-0609(2015)-05-1250-06
Al和Si对热浸Zn-Al-Mg合金镀层组织和耐腐蚀性的影响
贺志荣,刘继拓,解亚丹,周 超,刘 琳,戚云昊
(陕西理工学院 材料科学与工程学院,汉中 723001)
摘 要:
采用X射线衍射仪、扫描电子显微镜、能谱仪、透射电子显微镜、全浸腐蚀及中性盐雾腐蚀试验等方法,对比研究Zn-0.5Al-1.5Mg、Zn-2Al-1.5Mg和Zn-2Al-1.5Mg-0.3Si(质量分数,%)合金镀层的组织与性能,探讨Al和Si对热浸Zn-Al-Mg合金镀层组织和耐腐蚀性能的影响。结果表明:三合金镀层的组成相是Zn相、Al相和MgZn2相;Zn-0.5Al-1.5Mg合金镀层由块状富Zn相和晶界Zn+MgZn2二元共晶组织组成,Zn-2Al-1.5Mg合金镀层由块状富Zn相和晶界Zn+Al+MgZn2三元共晶组织组成,Zn-2Al-1.5Mg-0.3Si合金镀层由块状富Zn相、晶界Zn+Al+MgZn2三元共晶组织和针状相Mg2Si组成;Al、Mg和Si主要分布在晶界,Al和Si有细化镀层晶粒作用,添加Si能提高Zn-Al-Mg合金镀层的耐腐蚀性能。
关键词:
Zn-Al-Mg合金;Zn-Al-Mg-Si合金;热浸镀;镀层组织;耐腐蚀性能;
中图分类号:TG174.443 文献标志码:A
Effect of Al and Si on microstructure and corrosion resistance of hot dipping Zn-Al-Mg alloy coatings
HE Zhi-rong, LIU Ji-tuo, XIE Ya-dan, ZHOU Chao, LIU Lin, QI Yun-hao
(School of Materials Science and Engineering, Shaanxi University of Technology, Hanzhong 723001, China)
Abstract: The microstructure and properties of Zn-0.5Al-1.5Mg, Zn-2Al-1.5Mg and Zn-2Al-1.5Mg-0.3Si (mass fraction, %) alloy coatings were comparatively studied, and the effects of Al and Si on the microstructure and the corrosion resistance of hot dipping Zn-Al-Mg alloy coatings were investigated by X-ray diffraction, scanning electron microscope, energy dispersive spectrometer, transmission electron microscope, full immersion corrosion and neutral salt spray test. The results show that the composition phases of three alloy coatings are Zn, Al and MgZn2 phases. Zn-0.5Al-1.5Mg alloy coating is composed of bulk Zn-rich phase and Zn+MgZn2 eutectic structure at the grain boundary, Zn-2Al-1.5Mg alloy coating is composed of bulk Zn-rich phase and Zn+Al+MgZn2 eutectic structure at the grain boundary, Zn-2Al-1.5Mg-0.3Si alloy coating is composed of bulk Zn-rich phase, Zn+Al+MgZn2 eutectic structure at the grain boundary and acicular Mg2Si. The Al, Mg and Si distribute mainly at the grain boundary. The Al and Si possess the functions of refining the grain size. The corrosion resistance can be enhanced by the addition of Si in Zn-Al-Mg alloy coating.
Key words: Zn-Al-Mg alloy; Zn-Al-Mg-Si alloy; hot-dipping; coating microstructure; corrosion resistance
热浸镀锌技术能有效防止钢铁材料在大气中的腐蚀。自20世纪80年代以来,热浸镀锌合金及其浸镀技术得到了迅速发展,比利时冶金研究中心开发出了具有良好耐大气腐蚀性的Zn-5Al-0.05RE(高尔凡镀 层)合金镀层;随后的研究发现,在镀液中同时添加Al和Mg可显著提高镀层耐腐蚀性,又相继开发出了Zn-5Al-0.1Mg(深镀锌5%铝镀层)、Zn-0.2Al-0.5Mg (0.5%镁-锌镀层)、Zn-6Al-3Mg(6%铝-镁镀层)和Zn-11Al-3Mg-0.2Si(镀铝锌镁硅镀层)等合金镀层[1-3]。热浸镀锌技术的发展趋势是热浸镀合金多元化,新型Zn-Al-Mg系合金镀层已成为研究热点之一[4]。
目前,对新型Zn-Al-Mg系合金镀层研究已取得了重要进展。DUTTA等[5]研究发现,Zn-2.5Mg合金镀层中含有MgZn2相;Zn-0.5Mg-0.25Al合金镀层中含有二元和三元共晶混合物,其耐腐蚀性能好于Zn-Mg合金镀层。DEBRUYCKER等[6]研究发现,Zn-6Mg-3Al合金镀层含有Al相、MgZn2相Zn+Al+MgZn2三元共晶组织。同时发现,在Zn-5Al镀液中添加少量Mg后,镀层Zn+Al二元共晶组织粗化,Mg含量超过0.2%后,镀层生成Zn+Al+MgZn2三元共晶组织。YU等[7]在研究Zn-11Al-3Mg-0.2Si合金镀层时发现,该镀层主要含Zn相、Al相和Mg2Zn相,镀层表面存在雪花状枝晶、双六角组织以及层状和粒状共晶组织,其中雪花状枝晶为镀层主体结构;镀层形成过程为枝晶形核、长大→在枝晶附近形成MgZn2六方晶核→密排六方结构晶粒依靠MgZn2六方晶核长大→三元共晶组织凝固→枝晶分解为粒状共晶组织。SCHUERZ等[8-9]研究发现,在Zn-2Al-2Mg合金镀层中存在Zn+MgZn2二元共晶组织和Zn+Al+MgZn2三元共晶组织;若将该镀层进行短暂腐蚀,镀层表面会生成一层稳定且附着性较好的富Al层,该富Al层由Zn6Al2(CO3)(OH)16·4H2O组成。LEBOZEC等[10]将Zn-2Al-2Mg合金镀层置于CO2环境下的腐蚀结果表明,合金镀层腐蚀速率受环境影响明显,腐蚀产物主要由ZnO、Zn0.61Al0.39(OH)2(CO3)0.195· H2O和Zn5(OH)8Cl2·H2O组成。锌液中添加Al后,镀层会生成Fe-Al化合物层[11];添加Mg后,镀层会出现MgZn2相[7];通过控制Al、Mg、Si添加量可改善镀层组织和耐蚀性[12]。本文作者拟采用X射线衍射仪、扫描电子显微镜、能谱仪、透射电子显微镜和腐蚀试验等方法,通过对比研究Zn-0.5Al-1.5Mg、Zn-2Al-1.5Mg和Zn-2Al-1.5Mg-0.3Si(质量分数,%)合金镀层相组成、表面和截面组织及耐腐蚀性能,探讨Al和Si对热浸Zn-Al-Mg合金镀层组织和耐腐蚀性能的影响规律及机理,为研发耐蚀性能优异的新型Zn-Al-Mg和Zn-Al-Mg-Si合金镀层提供理论依据。
1 实验
1.1 试样制备
待镀材料为Q235钢板,将其切割、打磨、加工成尺寸为30 mm×30 mm×3 mm的试样。为方便浸镀,在试样侧面钻孔并挂铁丝。试样镀前处理工艺如下:1) 碱洗脱脂:150 g/L NaOH,温度为80 ℃,时间1 min;2) 水洗;3) 酸洗除锈:15%HCl加微量缓蚀剂,室温,时间1 min;4) 水洗;5) 电解活化助镀:电流大小为0.25~0.3 A,室温助镀2 min;6) 烘干:温度为120 ℃,时间:8~10 min。镀液成分为Zn-0.5Al-1.5Mg、Zn-2Al-1.5Mg和Zn-2Al-1.5Mg- 0.3Si。先将锌锭放入石墨坩埚中加热熔化,再倒入覆盖剂,然后将镁锭、铝锭压入坩埚底进行熔化并搅拌均匀。浸镀工艺参数:温度460~480 ℃、时间1 min、冷却方式为空冷15 s后水冷。锌液温度用探针式热电偶测量。
1.2 镀层组织和耐腐蚀性能分析
将镀层截面打磨、抛光,用2%硝酸酒精腐蚀,用丙酮超声波清洗,用XRD-7000S型X射线衍射仪、TESCAN Vega XMU型扫描电镜(SEM)、Oxford 7718型能谱仪(EDS)和JEM-2100HR高分辨率透射电子显微镜(TEM)观察分析镀层物相及表面、截面组织,用中性盐雾试验和全浸试验评价镀层耐腐蚀性。中性盐雾试验:将试样放入FQY015型气流式中性盐雾试验箱中,在35 ℃下用5%NaCl(体积分数)溶液周期性喷雾192h。全浸腐蚀试验:将试样挂在盛有5%Na2SO4(体积分数)溶液烧杯中,在35 ℃恒温箱中保温112 h。腐蚀试样再经过蒸馏水冲洗→饱和醋酸铵溶液擦洗→酒精清洗→吹干等程序后备用。
2 结果与讨论
2.1 镀层相组成
Zn-0.5Al-1.5Mg、Zn-2Al-1.5Mg和Zn-2Al-1.5Mg- 0.3Si(质量分数,%)合金镀层的相组成如图1所示。由图1可知,3种合金镀层主要由Zn相(密排六方hcp结构)、Al相(面心立方fcc结构)和MgZn2相组成,与文献[5-9]的结果相同。在Zn-Al-Mg合金镀层中随Al含量增加,或在Zn-Al-Mg合金镀液中添加Si后,MgZn2相的衍射峰逐渐减小,亦即MgZn2相数量减少,这可能与该合金中增加Al、Si含量后Zn相的相对量减少有关。
2.2 镀层表面组织
观察表明,本实验中制备的Zn-0.5Al-1.5Mg、Zn-2Al-1.5Mg和Zn-2Al-1.5Mg-0.3Si合金镀层表面均平整、无漏镀,镀层表面因Mg与空气发生反应形成氧化膜而有所发灰。图2所示为上述3种合金镀层表面扫描电镜(BSE)照片和EDS分析结果,由图2可以看出以下几点。
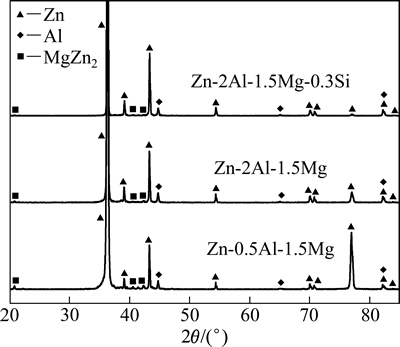
图1 合金镀层的相组成
Fig. 1 Phase compositions of Zn-0.5Al-1.5Mg, Zn-2Al- 1.5Mg and Zn-2Al-1.5Mg-0.3Si alloy coatings
1) 3种合金镀层中块状相为富Zn相;镀层晶界处Al、Mg和Si的含量高于块状相内部,这表明镀层中Zn未与Al、Mg、Si形成均匀固溶体。
2) Zn-Al-Mg合金镀层中,随Al含量增加,以及在该合金镀层中添加Si后,镀层晶粒细小、均匀化,其原因为分布在晶界处的Al对晶界迁移有阻碍作用,使镀层晶粒细化;同时,由于Mg、Al和Si熔点较高,冷却时先行凝固,对后凝固的Zn相产生了分隔作用,因此,亦使镀层晶粒细化。
3) Zn-0.5Al-1.5Mg合金镀层(见图2(a))由块状相和晶界处Zn+MgZn2二元共晶组织组成,其中,共晶组织系富Mg液相冷却至共晶温度时形成。
4) Zn-2Al-1.5Mg合金镀层(见图2(b))由块状相和晶界处Zn+Al+MgZn2三元共晶组织组成,其中,三元共晶组织系Al与Zn+MgZn2二元共晶组织反应而得。
5) Zn-2Al-1.5Mg-0.3Si合金镀层(见图2(c))由块状相、晶界处Zn+Al+MgZn2三元共晶组织和针状相组成。透射电子显微组织及电子衍射分析结果表明,针状相为直径约0.1 μm的Mg2Si(见图3),与文献[13]在Zn-10Al-3Mg-0.2Si合金镀层中给出的结果一致。
2.3 镀层截面组织
图4所示为Zn-0.5Al-1.5Mg、Zn-2Al-1.5Mg和Zn-2Al-1.5Mg-0.3Si合金镀层截面的BSE像及能谱线扫描结果。由图4可知,3种合金镀层厚度约20 μm,以Zn-2Al-1.5Mg-0.3Si合金镀层的晶粒最细,组织最致密。由能谱线扫描结果知,块状Zn相中Al、Mg和Si含量较低,晶界处Al、Mg和Si含量较高;钢基表面均出现Al含量的波动,说明钢基表面存在Fe-Al化合物相层。文献[14]中指出,Al含量较低时,Fe-Al化合物层随浸镀时间延长而消失。由图4(c)知,钢基表面除Al含量波动外,Si含量也出现了波动,说明在浸镀过程中Si元素在钢基表面聚集,使表面Si含量升高。HONDA等[13]研究表明,浸镀过程中,钢基体中Fe元素在四元Fe-Al-Zn-Si合金中比在三元Fe-Al-Zn合金中的扩散更困难,Fe-Al化合物相层较为致密,不影响镀层附着力。比较图4(b)和(c)中能谱线扫描结果可知,Zn-2Al-1.5Mg-0.3Si镀层表面Fe元素含量明显低于Zn-2Al-1.5Mg镀层,故在锌液中添加Si元素对钢基中Fe元素扩散有阻碍作用[15]。
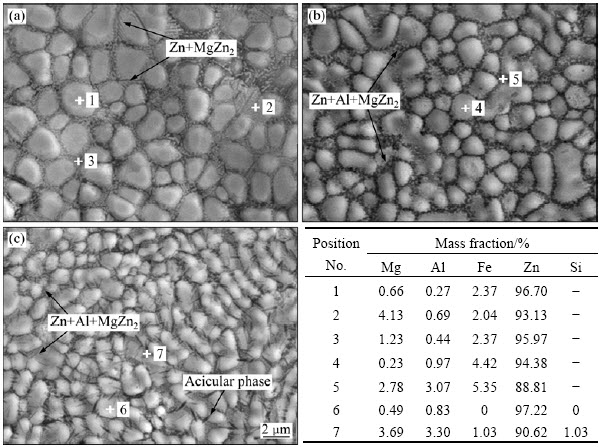
图2 Zn-0.5Al-1.5Mg、Zn-2Al-1.5Mg和Zn-2Al-1.5Mg-0.3Si合金镀层表面扫描电镜BSE像及EDS分析结果
Fig. 2 BSE images and EDS analysis of Zn-0.5Al-1.5Mg (a), Zn-2Al-1.5Mg (b) and Zn-2Al-1.5Mg-0.3Si (c) alloy coatings
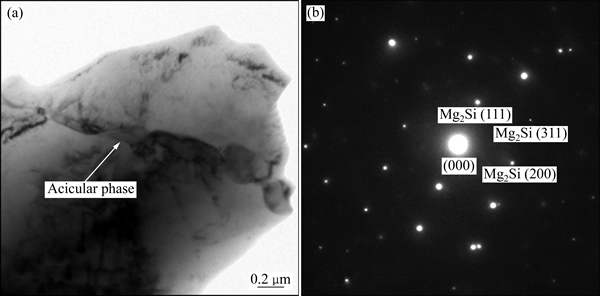
图3 Zn-2Al-1.5Mg-0.3Si合金镀层中针状相的透射电镜照片及其衍射花样
Fig. 3 TEM photograph (a) and electron diffraction pattern (b) of acicular phase in Zn-2Al-1.5Mg-0.3Si alloy coating

图4 Zn-0.5Al-1.5Mg、Zn-2Al-1.5Mg和Zn-2Al-1.5Mg-0.3Si合金镀层截面组织及各元素线扫描结果
Fig. 4 Microstructures and element line scanning results of Zn-0.5Al-1.5Mg (a), Zn-2Al-1.5Mg (b) and Zn-2Al-1.5Mg-0.3Si (c) alloy coatings
2.4 镀层耐腐蚀性能
图5所示为在中性盐雾和全浸腐蚀试验条件下Zn-0.5Al-1.5Mg,Zn-2Al-1.5Mg和Zn-2Al-1.5Mg-0.3Si合金镀层的耐腐蚀性能。由图5可以看出,3种合金镀层中,Zn-2Al-1.5Mg-0.3Si的耐蚀性最好,亦即添加Si元素能提高Zn-Al-Mg合金镀层的耐腐蚀性能。
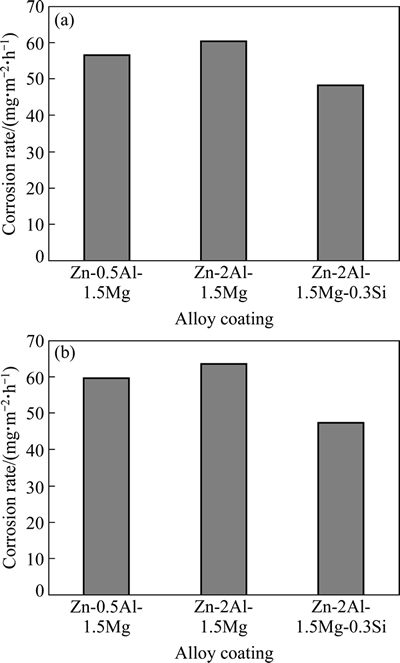
图5 Zn-0.5Al-1.5Mg、Zn-2Al-1.5Mg和Zn-2Al-1.5Mg-0.3Si合金镀层在中性盐雾和全浸腐蚀试验条件下的腐蚀速率
Fig. 5 Corrosion rates of Zn-0.5Al-1.5Mg, Zn-2Al-1.5Mg and Zn-2Al-1.5Mg-0.3Si alloy coatings in neutral salt spray (a) and full immersion (b) corrosion tests
其原因主要是由于在Zn-Al-Mg合金镀液中添加Si后,镀层组织均匀,晶粒细化,腐蚀反应受到抑制;MORIMOTO等[3]对不同合金镀层的电化学试验结果表明,添加Si元素可使镀层自腐蚀电流降低,耐腐蚀性能提高;Zn-2Al-1.5Mg-0.3Si合金镀层存在Mg2Si相,其电位比MgZn2相和Al相的低[16],发生腐蚀反应时,Mg2Si相作为阳极先发生溶解腐蚀,其中的高活性Mg元素会优先溶解,而不活泼的Si元素发生富集,促使Mg2Si相的电位正移并转换为阴极[17],从而使腐蚀电流降低、耐蚀性能提高。
Zn-0.5Al-1.5Mg和Zn-2Al-1.5Mg合金镀层的腐蚀速率也比较低。其原因主要是以下几点:1) 锌液中添加Mg元素后会促进镀层表面形成氧化镁薄膜,从而抑制其形成氧化锌薄膜;氧化锌薄膜对镀层无保护作用,氧化镁薄膜则能有效限制阴极反应;2) 添加Mg后,会在合金镀层形成均匀致密的共晶组织;3) Zn-Al-Mg合金镀层形成的腐蚀产物会填充在腐蚀裂缝,且腐蚀产物覆盖在镀层表面会形成致密的保护层。
3 结论
1) 热浸Zn-0.5Al-1.5Mg、Zn-2Al-1.5Mg和Zn-2Al-1.5Mg-0.3Si合金镀层的组成相是Zn相、Al相和MgZn2相,随Al、Si含量增加,MgZn2相数量减少。
2) Zn-0.5Al-1.5Mg合金镀层由块状富Zn相和晶界Zn+MgZn2二元共晶组织组成;Zn-2Al-1.5Mg合金镀层由块状富Zn相和晶界Zn+Al+MgZn2三元共晶组织组成;Zn-2Al-1.5Mg-0.3Si合金镀层由块状富Zn相、晶界Zn+Al+MgZn2三元共晶组织和针状相Mg2Si组成。
3) Al、Mg和Si主要分布在晶界,Al和Si有细化镀层晶粒作用,钢基表面Al和Si含量较高。
4) 添加Si元素能提高Zn-Al-Mg合金镀层的耐腐蚀性能。
REFERENCES
[1] SHINDO H, NISHIMURA K, OKADA T, NISHIMURA N, ASAI K. Developments and properties of Zn-Mg galvanized steel sheet “DYMAZINC” having excellent corrosion resistance[J]. Nippon Steel Technical Report, 1999, 79(2): 62-67.
[2] NISHIMURA K, KATO K, SHINDO H. Highly corrosion-resistant Zn-Mg alloy galvanized steel sheet for building construction materials[J]. Nippon Steel Technical Report, 2000, 81(2): 85-88.
[3] MORIMOTO Y, HONDA K, NISHIMURA KAZUMI, TANAKA S, TAKAHASHI A, SHINDO H, KUROSAKI M. Excellent corrosion-resistant Zn-Al-Mg-Si alloy hot-dip galvanized steel sheet “Super Dyma”[J]. Nippon Steel Technical Report, 2003, 87(1): 24-26.
[4] 刘继拓, 贺志荣, 何 应, 解 凯. 热浸Zn-Al-X合金镀层及工艺研究进展[J]. 材料导报, 2013, 27(3): 106-109.
LIU Ji-tuo, HE Zhi-rong, HE Ying, XIE Kai. Research progress of hot-dip Zn-Al-X alloy coatings and processes[J]. Materials Review, 2013, 27(3): 106-109.
[5] DUTTA M, HALDER A, SINGH S B. Morphology and properties of hot dip Zn-Mg and Zn-Mg-Al alloy coatings on steel sheet[J]. Surface & Coatings Technology, 2010, 205(7): 2578-2584.
[6] DEBRUYCKER E, ZERMOUT Z, DECOOMAN B. Zn-Al-Mg Coatings: Thermodynamic analysis and microstructure related properties[J]. Materials Science Forum, 2007, 539/543: 1276-1281.
[7] YU Kang-cai, LI Jun, LIU Xin, LI Jian-guo, XUE Xiao-huai. Microstructure of hot-dip galanized Zn-Al-Mg alloy coating[J]. Journal of Shanghai Jiao Tong University, 2012, 17(6): 663-667.
[8] SCHUERZ S, FLEISCHANDERL M, LUCKENEDER G H, PREIS K, HAUNSHCHMIED T, MORI G, KNEISSL A C. Corrosion behaviour of Zn-Al-Mg coated steel sheet in sodium chloride-containing environment[J]. Corrosion Science, 2009, 51(10): 2355-2363.
[9] SCHUERZ S, LUCKENEDER G H, FLEISCHANDERL M, MACK P, GSALLER H, KNEISSL A C, MORI G. Chemistry of corrosion products on Zn-Al-Mg alloy coated steel sheet[J]. Corrosion Science, 2010, 52(10): 3271-3279.
[10] LEBOZEC N, THIERRY D, ROHWERDER M, PERSSON D, LUCKENEDER G, LUXEM L. Effect of carbon dioxide on the atmospheric corrosion of Zn-Mg-Al coated steel[J]. Corrosion Science, 2013, 74: 379-386.
[11] 彭浩平, 苏旭平, 王建华, 李 智, 刘 亚. 热浸镀锌铝的界面反应机理[J]. 中国有色金属学报, 2012, 22(11): 3168-3175.
PENG Hao-ping, SU Xu-ping, WANG Jian-hua, LI Zhi, LIU Ya. Interface reaction mechanism for galvanizing in Zn-Al baths[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(11): 3168-3175.
[12] 贺志荣, 何 应, 刘继拓, 解 凯. Al和RE对Zn-Al合金镀层组织和耐蚀性的影响[J]. 中国有色金属学报, 2014, 24(8): 2020-2025.
HE Zhi-rong, HE Ying, LIU Ji-tuo, XIE Kai. Effects of Al and RE on structure and corrosion resistance of Zn-Al alloy coatings[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(8): 2020-2025.
[13] HONDA K, USHIODA K, YAADA WATARU. Influence of Si addition to the coating bath on the growth of the Al-Fe alloy layer in hot-dip Zn-Al-Mg alloy-coated steel sheets[J]. ISIJ International, 2011, 51(11): 1895-1902.
[14] INAGAKI J. Studies on Zn-Fe alloying reactions in hot-dip galvanizing and galvannealing process[J]. Surface Technology, 2000, 51(6): 574-579.
[15] YIN Fu-cheng, ZHAO Man-xiu, LIU Yong-xiong, HAN Wei, LI Zhi. Effect of Si on growth kinetics of intermetallic compounds during reaction between solid iron and molten aluminum[J]. Transaction of Nonferrous Metals Society of China, 2013, 23(2): 556-561.
[16] LI J F, ZHENG Z Q, LI S C, CHEN W J, REN W D, ZHAO X S. Simulation study on function mechanism of some precipitates in localized corrosion of Al alloys[J]. Corrosion Science, 2007, 49: 2436-2449.
[17] ZENG Feng-li, WEI Zhong-ling, LI Jin-feng, LI Chao-xing, TAN Xing, ZHANG Zhao, ZHENG Zi-qiao. Corrosion mechanism associated with Mg2Si and Si particles in Al-Mg-Si alloys[J]. Transaction of Nonferrous Metals Society of China, 2011, 21(12): 2559-2567.
(编辑 李艳红)
基金项目:陕西省科技统筹创新工程计划(2011KTDZ01-03-06);陕西省大学生创新创业训练计划(1626);陕西理工学院研究生创新基金资助项目(SLGYCX1432);陕西理工学院研究生教改项目资助(SLGYJG1404)
收稿日期:2014-06-26;修订日期:2015-02-14
通信作者:贺志荣,教授,博士;电话:0916-2641895;E-mail:hezhirong01@163.com
摘 要:采用X射线衍射仪、扫描电子显微镜、能谱仪、透射电子显微镜、全浸腐蚀及中性盐雾腐蚀试验等方法,对比研究Zn-0.5Al-1.5Mg、Zn-2Al-1.5Mg和Zn-2Al-1.5Mg-0.3Si(质量分数,%)合金镀层的组织与性能,探讨Al和Si对热浸Zn-Al-Mg合金镀层组织和耐腐蚀性能的影响。结果表明:三合金镀层的组成相是Zn相、Al相和MgZn2相;Zn-0.5Al-1.5Mg合金镀层由块状富Zn相和晶界Zn+MgZn2二元共晶组织组成,Zn-2Al-1.5Mg合金镀层由块状富Zn相和晶界Zn+Al+MgZn2三元共晶组织组成,Zn-2Al-1.5Mg-0.3Si合金镀层由块状富Zn相、晶界Zn+Al+MgZn2三元共晶组织和针状相Mg2Si组成;Al、Mg和Si主要分布在晶界,Al和Si有细化镀层晶粒作用,添加Si能提高Zn-Al-Mg合金镀层的耐腐蚀性能。


