
J. Cent. South Univ. (2021) 28: 1324-1332
DOI: https://doi.org/10.1007/s11771-021-4705-y
Effect of reductant sodium bentonite content and reaction temperature in sponge iron production from composite pellets
Ilker KARA
Department of Medical Services and Techniques, Eldivan Medical Services Vocational School,Cankiri Karatekin University, Cankiri 18100, Turkey
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract:
In recent years, composite pellet production with added reductant has been developed instead of traditional iron production. Composite pellets produced by the addition of appropriate proportions of reductant produce sponge iron in the reductant melting process at high temperatures. The elements created in the structure by pellet production directly affect the quality of the product obtained by determining the chemical composition and the appropriate reaction temperature. In this study, sponge iron ore concentrate (scale) and reductant (coke coal dust and sodium bentonite) were mixed at certain proportions to produce composite pellet samples; the effects of addition rate of the reductant material of sodium bentonite (1 wt%-4 wt%) and variation in reaction temperature (900-1200 °C) on the metallization and compressive strength properties of the produced composite pellet samples were investigated. The analysis results show that the highest compressive strength is obtained from pellet samples produced with 3% sodium bentonite at 1100 °C. Additionally, SEM-EDS analysis results of the samples show that the morphologic structure has much lower porosity rates compared to samples produced under the other conditions which makes the samples denser and increases the metallization properties.
Key words:
solid waste; reduction; iron oxides; pellets; sponge iron;
Cite this article as:
Ilker KARA. Effect of reductant sodium bentonite content and reaction temperature in sponge iron production from composite pellets. [J]. Journal of Central South University, 2021, 28(5): 1324-1332.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-021-4705-y1 Introduction
The term composite pellet is generally used for pellets containing fine iron oxide and carbonaceous material (coal, coke and charcoal). Composite pellets have advantages like high reaction rate linked to the good mixing of oxide and carbon and the ability to use nonsmoking coal [1]. When composite pellets containing iron oxide and carbon are heated to high temperatures, the sponge iron produced by reduction of iron oxides may partly or fully melt linked to the temperature and the degree of carburization of the product [2]. During the process, variation in the pellet structure is linked to many factors [2], including the reaction temperature of the furnace, amount of reductant material added, pellet size, duration spent in the furnace and the amount of unwanted contaminant material in the mixture [3].
Composite pellets containing sponge iron ore, coal and sodium bentonite as binder reacted at high temperatures for a variety of durations and investigated by MATSUMURA et al [4]. Sponge iron is produced by the direct reduction of iron oxide ore, pellets or concentrates to metallic iron with the help of a reducing gas or solid fuel in a rotary furnace or shaft furnace at approximately 1000 °C without melting. The reduced product, which contains oxide gang in specific proportions and has a metallic Fe content of 80%-85%, is called sponge iron because of its spongy appearance [5].
The structure of sponge iron is determined by the raw material properties and production method used. As a result of the iron ore reduction process, sponge iron, also called direct reduced iron (DRI), is obtained [6]. The sponge iron obtained as a result of this process contains very small amount of impurities and the total iron content is above 90% in general; and the remaining part contains up to 2.5% carbon and unreduced iron oxides and gang. Many steel producers use sponge iron as charging material in electric arc furnaces. Nowadays, due to the increased scrap prices, sponge iron is used in place of scrap in electric arc furnaces to obtain high quality steel [7-9]. In addition to this, sponge iron is used in many areas such as basic oxygen furnaces, iron foundries and ladle metallurgy.
Reduction reactions of iron oxide with H2/H2O or CO/CO2 gas mixtures are predicted to occur in the following manners:

The free iron released after the reaction reacts with the carbon and carbon compounds present in the medium during the cooling process to form Fe3C (iron carbide).
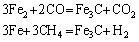
The resulting mixture of Fe-Fe3C and iron oxide is called DRI or sponge iron. Scale is an iron oxide layer formed during reduction on the steels, slabs and billet surfaces produced in rolling mills, continuous casting plants and annealing furnaces. This material, which contains approximately 70% iron, has been considered waste in iron-steel plants for many years. The binders are used both in the agglomeration of the ore after crushing and grinding processes in the iron and steel industry as well as in the palletization or briquetting of iron oxides in direct reduced iron production [10-12]. The binder is used for two main purposes: 1) to retain free water in the ore concentrate/iron oxide and 2) to prevent the dissolution of pellet or briquette.
One of the most widely used binders in the iron and steel industry due to its low cost and abundance is sodium bentonite [13]. However, there are pelletizing studies carried out with organic binders to improve its properties.
In the new plants established in recent years, iron oxide layer has started to be used as a new alternative in the production of sponge iron. Recycling of this material will be both economically and environmentally beneficial [1].
The management and recycling of metallic waste material like scale will be beneficial for the costs of transporting waste iron oxide to storage sites and disposal, the sustainability required for iron production, environmental pollution and investments in the future from an economic aspect. However, the efficiency of these processes in terms of energy and costs may be a topic of debate at times. Typical production costs for sponge iron using an electric arc furnace are given in Tables 1 and 2 [14, 15].
Table 1 Cost of pelleting
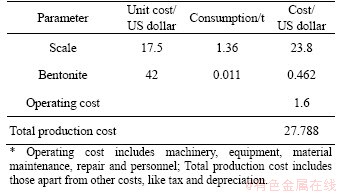
Table 2 Sponge iron production cost
Sponge iron production from composite pellets appears to be a good alternative due to problems related to cost of raw materials and input supply. An industrial common life implementation created with this approach lowers production costs and provides a competitive advantage for system partners.
2 Pelletizing and agglomeration mechanism in pelletizing
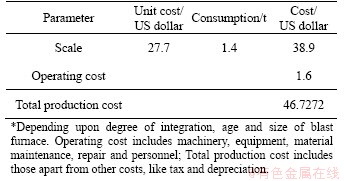
The pelletizing process consists of 1) mixing the concentrated or iron and steel product waste (oxide layer, etc.), coal dust, binder with water at appropriate ratios, 2) the agglomeration process in which the mixture is adhered, and 3) the annealing process of the sample. The pellets produced must be between 9-16 mm in diameter and must have sufficient strength. The pellets are used as raw materials in blast furnaces and direct reduction processes. In other words, some of the pellets are the raw materials in sponge iron production, which can be used instead of scrap. In addition, the pellets of very clean concentrates of iron ores are used directly in the production of iron dust for reduction metallurgy (Figure 1).
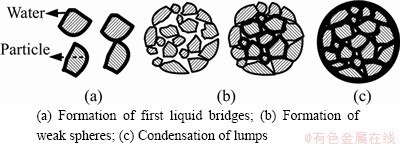
Figure 1 Coating of ore or scale fragment with water film:
The capillary-bridging force is effective in the wet sphere formation. Optimum sphere formation occurs when all the pores in the sphere are filled with liquid. The bonding force between the particle surface and water, particle surface properties, mechanical pressures, collision forces, and mechanical strength of the particles are the determining factors in the formation of wet spheres.
In the formation of spheres, the tensile forces of the particles change according to the filling rate of the capillary cavities of the particles with water. They are formed by the combined effects of binding, capillary bridging and surface tension depending on the fill rate of the capillary cavities with water.
In agglomeration with liquid, it is assumed that the optimum strength is achieved when the gaps between the ore particles are almost completely filled with liquid (for example water) and the liquid surfaces of water-filled capillaries are concave on agglomerate surfaces as shown in Figure 2. Capillary stress is the main cause of wet pellet strength. The bridge binding is practically no longer present. Under certain conditions, however, depending on the size of the particle, it may remain effective on the pellet surface. At room temperature, scales formed by oxidation have smaller porosity. These pores can be easily deformed, and can be shrunk to form very coherent tight surfaces in appropriate conditions (see Figure 2(c)).
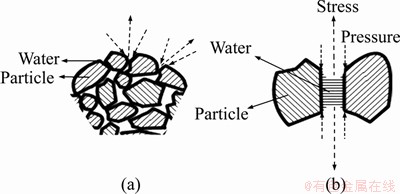
Figure 2 Capillary tension and pressure force on pellets (a) and between two particles (b)
As can be seen in the Bauer-Glaessner diagram and the Boudouard curves, metallic iron reduction of iron oxides is only possible above 705°C [16]. A Wüstite scale layer is formed when it falls below 700°C. The Wüstite layer (FeO) has the lower oxygen content compared to other iron oxides and is also called metal-like layers. With the temperature rising to 700°C, Wüstite layer begins to form instead of magnetite. Under 800°C, iron oxides cannot be reduced to metallic iron, but can be converted into hematite magnetite. In this work, the palletization process was subjected to the reduction process at 900-1200°C.
With the reduction melting process, the current production steps in technology used for the purpose of composite pellet production at the moment are shortened with the aim of being both cheap and less polluting, by using raw material with limited use at the highest levels based on available technology.
With this aim, much research has been performed to date to determine the ideal conditions for the reduction melting process and to increase the metallization and compressive degrees of composite pellets produced and the numbers of these studies continue to increase [17].
The study offers a systematic approach for production of sponge iron with the reduction melting process. Within the scope of the study, composite pellet samples were produced with four different sodium bentonite addition rates and four different reaction temperatures. These variations were implemented in order to investigate the effect on the metallization and compressive features of the pellets.
3 Materials and methods
Solid iron oxide waste (scale) obtained from Kardemir Inc., Turkey was left in the “Incubator (MST-120D)” brand drying furnace at Iron and Steel Institute, Karabuk University for 6 h at 120 °C to remove moisture from the samples. In order to reduce the grain size of the scale and coal dust, they were milled. In the scale and coal dust grinding process, first the grinding mill was prepared by cleaning with alcohol and sea sand (SiO2). The cleaning process was carried out again before processing each sample. The scales were milled at 1500 r/min for 120 s. And the coal dusts were milled at 1500 r/min for 30 s. The samples were brought to 100 μm in diameter [18].
Pelletizing process is used to obtain pellets which will be used in the industry by mixing coal dust, sodium bentonite and solid waste scale in iron and steel plants. For the pelletization process, the pellet machine at Faculty of Mines, Istanbul Technical University was used. The pellets consist of 73% scale (0.25-1 mm particle size), 26% coal dust (0.05-0.1 mm particle size) and 1% binder (sodium bentonite, 0.25-1 mm particle size). Sodium bentonite ratio was then increased by 1% and 2%, for 3% and 4% binder mass ratios respectively. While increasing the sodium bentonite ratio, the scale ratio was reduced with the same rate (72% scale, 2% binder; 71% scale, 3% binder; 70% scale, 4% binder). For the pellet compositions, 600 g blends of scales were prepared, and the mixtures were separately pelletized by adding 10% water in the pelletizer. The sizes of the pellets produced were 8-20 mm, which is the ideal pellet size provided.
For the wet strength measurement of the produced pellets, 4-5 new pellets selected were dropped from 50 cm height to the hard floor. In this test, if a pellet does not break up 3 to 4 times in a row, it will pass the wet strength test and it will be considered to have sufficient wet strength value for subsequent processes. In the tests, it was observed that the pellets prepared in all compositions reached at least 5 drops. Samples for the compression test were prepared using 60D sander. For the SEM-EDS analysis, however, samples were prepared by using the sanders of 60D and 540D, respectively [19].
It was observed that smaller-sized particles are required for large-sized particles to become spherical. Pellets of the desired size are formed with the binding of the first cores formed. Produced pellets were reduced by the heat treatment furnace (PLF 130/45) at Iron and Steel Institute, Karabuk University at 900-1200 °C for 120 min. In this way, sponge irons with four different temperature parameters were produced from the scales obtained from solid iron oxide.
The reduction reaction from iron oxides to iron is as follows:
Fe3O4+4C=3Fe+4CO
Fe2O3+3C=2Fe+3CO
FeO+C=Fe+CO
In metallographic process, Tegramin 30 grinding machine Iron and Steel Institute, in Karabuk University was used to make the sponge iron produced ready for SEM-EDS analysis. In compression strength test, compression strength of the sponge iron produced was measured by “Zwick/Roell Z600 Universal Testing Machine”. SEM device was used in order to see the internal structure of the produced pellets and to make their elemental analysis. The measurements of the samples used for the measurements were made under the same conditions. First, the sample containers were mechanically cleaned before each measurement. As a second step, they were cleaned with ionized water and dried with dry nitrogen gas.
4 Results and discussion
In the reduction process, the reaction temperatures, type and density of the elements comprising the pellets affect the structure formation in pellets in solid melt form and play an important role in the creation of metallization and compressive strength features of the composite pellets produced. The metallization and compressive strength features of composite pellet samples obtained under controlled experimental conditions were investigated linked to variations in the reduction process parameters of sodium bentonite addition rate and reaction temperature.
4.1 Analysis of metallization
Metallization analyses investigate the internal structure of samples and can determine geometric structure, porosity and chemical properties [20, 21]. The metallization investigation of composite pellet samples obtained with reduction experiments used the SEM/EDS method [22-24]. With this aim, composite pellet samples produced with 3% sodium bentonite addition at 900-1200 °C were analyzed with SEM/EDS (Figure 3). SEM images used back-scatter electrons and the metal phase was observed as light contrast. Dark phases in the structures show unreduced oxide phases, contaminant atoms and porosity.
When the reaction temperature fell to 900 °C, the pellet samples had particles with geometric structure and high porosity (Figure 3). This situation is due to events caused by thermal activation not occurring as they should in the structures of the pellet samples produced at these temperatures. As a result, porosity formation and interstice atom movements were limited, so granulation occurred in the structure. These samples with weak and high porosity may be easily fragmented, which is an unwanted situation for composite pellet production. According to EDS analysis, the structure was observed to contain oxide structures comprising Fe, Al, Mn, Si, Cu and Ca elements. Additionally, high intensity was encountered for the C peak, showing that there is unburned carbon in the structure. This situation shows that the reduction amounts for pure carbon in the scale and additionally in the pig iron were low; in other words, the reduction process did not occur at desired levels.
Composite pellets produced at 1000 °C have particles in the geometric structure which have aggregated in groups, and porosity largely seems to be lost with the increase in temperature. The cavities in the structure of the samples have largely reduced and they are denser. According to EDS analysis, the structure contains oxide structures of Fe, Al, Mn, Si, Cu and Ca elements, but the intensities of the peaks for these elements were observed to reduce. Additionally, a reduction in intensity was observed for the C peak. This situation shows an increased amount of reduction of pure carbon in scale and additionally carbon in pig iron (Figure 4).
Composite pellet samples produced at 1100 °C have particles in the geometric structure aggregated into very tight groups, with nearly all porosity lost. This situation means that the structure is very sturdy and pellets have been were obtained with the desired features. According to EDS analysis, the intensities of peaks were observed to be very reduced along with oxide structures comprising Fe, Al, Mn, Si, Cu and Ca in the structure. Additionally, the C peak reduced and was close to the intensity of the Fe peak (Figure 5). This situation shows that pure carbon in waste scale and carbon in pig iron were reduced; in other words, the reduction process occurred without errors.
The structure of composite pellets produced at 1200 °C appeared to be more brittle (Figure 6). As the temperature increased a lot, thin fractures formed in the geometric structure of the pellets. According to EDS analysis, the oxide structures comprising Fe, Al, Mn, Si, Cu and Ca, and the peak intensity appears to have increased compared to composite pellets produced at 1100 °C. Additionally, an increase in the intensity for the C peak was observed, and this situation shows that the amount of reduction of pure carbon in scale and carbon in pig iron was lower compared to the composite pellets produced at 1100 °C.
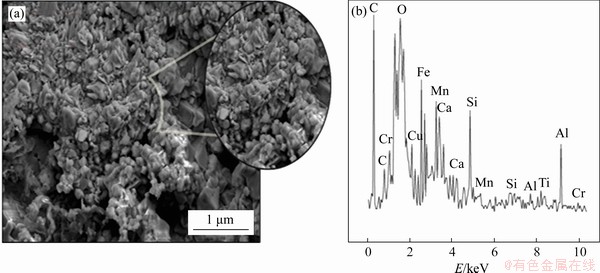
Figure 3 SEM images (a) and EDS analysis (b) of composite pellets produced at 900 °C with 3% sodium bentonite content
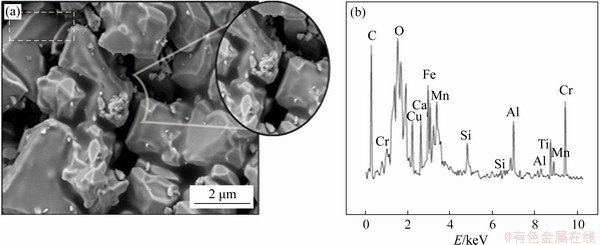
Figure 4 SEM images (a) and EDS analysis (b) of composite pellets produced at 1000 °C with 3% sodium bentonite content
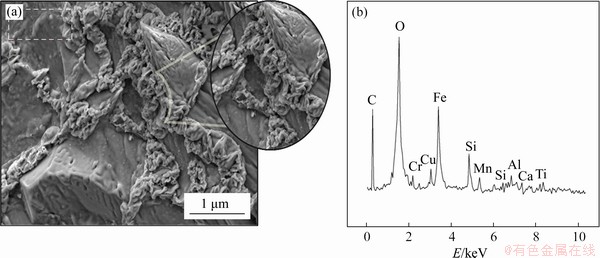
Figure 5 SEM images (a) and EDS analysis (b) of composite pellets produced at 1100 °C with 3% sodium bentonite content
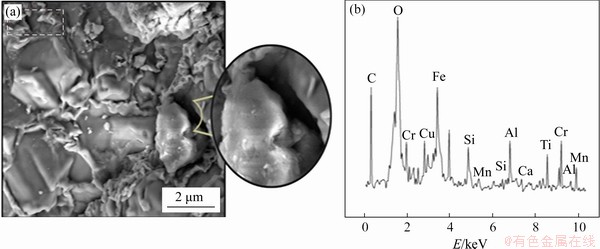
Figure 6 SEM images (a) and EDS analysis (b) of composite pellets produced at 1200 °C with 3% sodium bentonite content
The metallization level in the composite pellet samples represents how much of the total Fe element contained in the structure has been reduced. To compare the variation in Fe elements in the composite pellet samples produced with 3% addition at 900-1200 °C, all EDS spectra are given as a single graph in Figure 7. Samples produced at 900 °C had the lowest peak intensity for Fe, while the peak intensity for the Fe element increased with increasing temperature and reached the highest level at 1100 °C. Pellets produced at 1200 °C appeared to have second highest intensity for Fe element peaks. From these results, the best metallization level was observed for samples produced at 1100 °C, with the lowest metallization levels observed for samples produced at 900 °C.
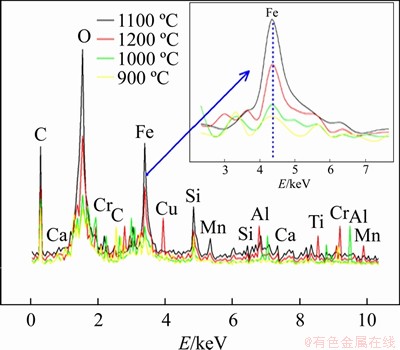
Figure 7 EDS spectra for composite pellets produced at 900-1200 °C with 3% sodium bentonite content
4.2 Compressive strength analysis
Compressive strength represents the resistance that a material displays to change in the applied force. The compressive strength test provides important information about properties like the internal structure of the material and metallization levels, so pellet production studies frequently use this method [25]. As a result, the compressive strength tests were applied to the composite pellets to determine mechanical resistance, internal structure and metallization level (Figure 8). Compressive strength tests were completed by applying compressive force to pellet samples placed between compression plates in a compression machine. Compressive strength force is found with the aid of σb,g=Fi/Ai where Ai is the true section of the pellet and Fi represents applied force. Coal dust and sodium bentonite were used as reductants in the composite pellet samples. The amount of coal dust was fixed, while the sodium bentonite addition rate was varied (1 wt%-4 wt%) and the effect on the reduction process for samples produced at 900-1200 °C was investigated with the compressive strength tests (Figure 8).
The compressive strength was at low levels for samples with 1%-4% sodium bentonite produced at 900 °C (Figure 8). Though the sodium bentonite addition rate increased in samples produced at 900 °C, reduction had limited effect and metallization levels of the pellet samples did not reach desired levels so strength remained low. For samples produced at 1000 °C, samples with 1% sodium bentonite content had low strength properties, while the metallization level of the samples increased with increased addition rate and it was observed that strength values reached desired levels. The best strength value for samples produced at this temperature was observed for 3% addition, with the strength value observed to lower slightly at 4% addition. Of the samples produced at 1100 °C, samples with 1% sodium bentonite content had low strength properties, while metallization levels had a tendency to increase with the increase in content. The 3% sodium bentonite sample reached the highest reduction levels and this sample had the best strength. When addition rate reached 4%, the strength value appeared to fall slightly.
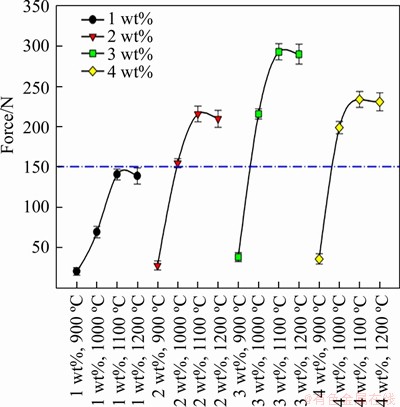
Figure 8 Compressive strength values for composite pellets produced at 900-1200 °C with 1%-4% sodium bentonite content
For samples produced at 1200 °C, 1% sodium bentonite content had increased strength properties, but did not reach desired levels. With increasing temperature, the reduction level increased with sodium bentonite up to 3%, while strength features appeared to reduce slightly for samples with 4% sodium bentonite. It appeared that the samples with 1%-4% sodium bentonite produced at 1100 °C had higher strength properties compared to samples produced at 1200 °C. This situation may be due to the increasing brittleness of the samples produced at higher temperatures being negatively reflected in the strength properties.
5 Conclusions
In this study, a semi-finished product, sponge iron is produced in a single process from scale, which is a waste containing high iron content, in a shorter period of time more economically than other alternatives on the market, using less energy and causing less harm to the environment. It is important to determine the characteristic element compositions that make up the morphological structures of the pellets obtained from solid waste scale samples. The analysis results show that the formation of pellet samples depends on the chemical composition and annealing temperature. The results show that the formation and strength of DRI structure depends on the concentrations and annealing temperature of the structure.
As a result of the SEM-EDS analysis, it is observed that DRI obtained is satisfactory with intended purity, and it is found that microstructure also contains pores and some raw material-originated impurities as well as the unreduced iron oxide caused by the annealing temperature and amount of reducing agent. The metallization and compressive strength features of the composite pellet samples are investigated linked to the reduction process parameters of sodium bentonite addition rate and reaction temperature. The highest compressive strength value is obstained for samples produced at 1100 °C reaction temperature with 3% sodium bentonite contribution. Additionally, these samples have the lowest levels of porosity and highest levels of metallization. From these results, the most appropriate conditions are identified as 3% sodium bentonite and 1100 °C reaction temperature for production of composite pellet samples. The pellets obtained can be used in place of scrap or in conjunction with scrap in steel production, as a clean raw material with very-low impurity and known structure, in a basic oxygen furnace or in an electric arc furnace. In addition, it is believed that it can be considered alternative raw material instead of scrap and pig iron in the pig casting and steel foundries.
Conflict of interest
Ilker KARA declare that there is no conflict of interest.
References
[1] DAS B, PRAKASH S, REDDY P S R, MISRA V N. An overview of utilization of slag and sludge from steel industries [J]. Resources, Conservation and Recycling, 2010, 50(1): 40-57. DOI: 10.1016/j.resconrec.2006.05.008.
[2] PENG J, TANG M T, PENG B, YU, D, KOZINSKI J A, TANG C B. Heating and melting mechanism of stainless steelmaking dust pellet in liquid slag [J]. Journal of Central South University of Technology, 2007, 14(1): 32-36. DOI: 10.1007/s11771-007-0007-2.
[3] PARK J W, AHN J C, SONG H, PARK K, SHIN H, AHN J S. Reduction characteristics of oily hot rolling mill sludge by direct reduced iron method [J]. Resources, Conservation and Recycling, 2002, 34(2): 129-140. DOI: 0.1016/S0921-3449(01)00098-2.
[4] MATSUMURA T, TAKENAKA Y, SHIMIZU M, NEGAMI T, KOBAYASHI J, URAGAMI A. Direct production of molten iron from carbon composite iron ore pellet [J]. La Revue de Métallurgie-CIT, 1998, 95(3): 341-352. DOI: 10.1051/metal/199895030341.
[5] JUNG W G. Recovery of tungsten carbide from hard material sludge by oxidation and carbothermal reduction process [J]. Journal of Industrial and Engineering Chemistry, 2014, 20(4): 2384-2388. DOI: 10.1016/j.jiec.2013.10.017.
[6] BOECHAT F O, de CARVALHO R M, TAVARES L M. Simulation of mechanical degradation of iron ore pellets in a direct reduction furnace [J]. KONA Powder and Particle Journal, 2018, 35: 216-225. DOI: 10.14356/kona.2018009.
[7] JOHANSSON A, HOLAPPA L. From megaplants to mini-mills-a trend in steelmaking-a prospect for papermaking [J]. Resources, Conservation and Recycling, 2004, 40(2): 173-183. DOI: 10.1016/S0921-3449(03)00069-7.
[8] OZAWA L, SHEINBAUM C, MARTIN N, WORRELL E, PRICE L. Energy use and CO2 emissions in Mexico’s iron and steel industry [J]. Energy, 2002, 27(3): 225-239. DOI: 0.1016/S0360-5442(01)00082-2.
[9] KIRSCHEN M, RISONARTA V, PFEIFER H. Energy efficiency and the influence of gas burners to the energy related carbon dioxide emissions of electric arc furnaces in steel industry [J]. Energy, 2009, 34(9): 1065-1072. DOI: 10.1016/j.energy.2009.04.015.
[10] CETINKAYA S, EROGLU S. Thermodynamic analysis and reduction of tin oxide with methane [J]. International Journal of Mineral Processing, 2012, 110: 71-73. DOI: 10.1016/ j.minpro.2012.04.005.
[11] LI G H, YOU Z X, ZHANG Y B, RAO M J, WEN P D, GUO Y F, JIANG T. Synchronous volatilization of Sn, Zn, and As, and preparation of direct reduction iron (DRI) from a complex iron concentrate via CO reduction [J]. JOM, 2014, 66(9): 1701-1710. DOI: 10.1007/s11837-013-0852-4.
[12] ZARE G A, VALIPOUR M S, VAHEDI S M, SOHN H Y. A review on the modeling of gaseous reduction of iron oxide pellets [J]. Steel Research International, 2020, 91(1): 1900270. DOI: 10.1002/srin.201900270.
[13] CHUN T, LONG H, DI Z, MENG Q, WANG P. Preparation of direct reduction sponge iron (DRI) using pyrite cinder containing nonferrous metals [J]. High Temperature Materials and Processes, 2017, 36(10): 971-978. DOI: 10.1515/htmp-2016-0072.
[14] STALHED J L. Sponge iron in electric arc furnaces [J]. JOM, 1957, 9(2): 246-249. DOI: 10.1007/BF03398481.
[15] HAVEMANN H A. Direct iron ore reduction for Asia [J]. Indian Construction News, 1959(8): 260-272. http://eprints. nmlindia.org/3059/1/ 260-272.PDF.
[16] PINEAU A, KANARI N, GABALLAH I. Kinetics of reduction of iron oxides by H2: Part I: Low temperature reduction of hematite [J]. Thermochimica Acta, 2006, 447(1): 89-100. DOI: 10.1016/j.tca.2005.10.004.
[17] ZHU D Q, MENDES V, CHUN T J, PAN J, LI Q H, LI J, QIU G Z. Direct reduction behaviors of composite binder magnetite pellets in coal-based grate-rotary kiln process [J]. ISIJ International, 2011, 51(2): 214-219. DOI: 10.2355/ isijinternational.51.214.
[18] KHAIDUROVA A A, KONOVALOV P N, KONOVALOV N P. Microwave treatment of a brown coal concentrate from mugunsk coal for the manufacture of sponge iron. [J]. Solid Fuel Chemistry, 2008, 42(2): 120-122. DOI: 10.3103/ S0361521908020122.
[19] GAO S, BROWN B, YOUNG D, SINGER M. Formation of iron oxide and iron sulfide at high temperature and their effects on corrosion [J]. Corrosion Science, 2018, 135(1): 167-176. DOI: 10.1016/j.corsci.2018.02.045.
[20] ESPER F J, EXNER H E, METZLER H. The correlation between raw materials, preparation conditions, and properties of sintered iron [J]. Powder Metallurgy, 1975, 18(35): 107-123. DOI: 10.1179/pom.1975.18.35.008.
[21] van WYK S, NEOMAGUS H W J P, BUNT J R, EVERSON R C. Coal reactivity and selection for solid-based pre-reduction of sponge iron [J]. International Journal of Coal Preparation and Utilization, 2020, 40(4, 5); 233-246. DOI: 10.1080/19392699.2017.1384729.
[22] ZHANG Y B, LI G H, JIANG T, GUO Y F, HUAN Z C. Reduction behavior of tin-bearing iron concentrate pellets using diverse coals as reducers [J]. International Journal of Mineral Processing, 2012, 110: 109-116. DOI: 10.1016/ j.minpro.2012.04.003.
[23] SEN R, PANDEL U. Closed crucible reduction of lump powdered mill scale or iron ore by coal: The sequential methodology and mechanism for optimization of process parameters [J]. Advanced Powder Technology, 2020, 31(9): 3760-3773. DOI: 10.1016/j.apt.2020.07.017.
[24] NAYAK D, RAY N, DASH N, RATH S S, BISWAL S K. Reduction behavior of Odisha sands complex, India ilmenite-coke composite pellets [J]. Journal of Central South University, 2020, 27(6): 1678-1690. DOI: 10.1007/s11771-020-4399-6.
[25] YU W, SUN T C, LIU Z Z, KOU J, XU C Y. Study on the strength of cold-bonded high-phosphorus oolitic hematite-coal composite briquettes [J]. International Journal of Minerals, Metallurgy, and Materials, 2014, 21(5): 423-430. DOI: 1 0.1007/s12613-014-0925-6.
(Edited by FANG Jing-hua)
中文导读
复合球体生产海绵铁过程中还原剂膨润土钠含量和反应温度的作用
摘要:近年来,添加还原剂的复合球体铁生产逐步代替传统的铁生产。在高温下,加入适当比例的还原剂,在其熔化过程中产生海绵铁。通过确定化学成分和适当的反应温度可以获得产品,而球体结构在生产中产生的元素直接影响产品的质量。本研究将海绵铁矿石精矿(等级)和还原剂(焦煤尘和膨润土钠)按一定比例混合制备复合球体样品,研究了膨润土钠还原剂的添加量(1wt%~4wt%)和反应温度变化(900~1200 ℃)对制备复合球体样品金属化和抗压强度性能的影响。分析结果表明,在1100 °C下用3%膨润土钠制备的复核球体样品的抗压强度最高。此外,SEM-EDS分析结果表明,与其他条件下生产的样品相比,本研究制备的材料孔隙率要低得多,样品更致密,其金属化性能得到了提高。
关键词:固体废物;还原量;氧化铁;球体;海绵铁
Received date: 2020-05-23; Accepted date: 2020-11-14
Corresponding author: Ilker KARA, PhD, Assistant Professor; Tel: +90-376-311-2042; E-mail: karaikab@gmail.com; ORCID: https:// orcid.org/0000-0003-3700-4825
Abstract: In recent years, composite pellet production with added reductant has been developed instead of traditional iron production. Composite pellets produced by the addition of appropriate proportions of reductant produce sponge iron in the reductant melting process at high temperatures. The elements created in the structure by pellet production directly affect the quality of the product obtained by determining the chemical composition and the appropriate reaction temperature. In this study, sponge iron ore concentrate (scale) and reductant (coke coal dust and sodium bentonite) were mixed at certain proportions to produce composite pellet samples; the effects of addition rate of the reductant material of sodium bentonite (1 wt%-4 wt%) and variation in reaction temperature (900-1200 °C) on the metallization and compressive strength properties of the produced composite pellet samples were investigated. The analysis results show that the highest compressive strength is obtained from pellet samples produced with 3% sodium bentonite at 1100 °C. Additionally, SEM-EDS analysis results of the samples show that the morphologic structure has much lower porosity rates compared to samples produced under the other conditions which makes the samples denser and increases the metallization properties.


