
Growth mechanism of Cu nanopowders prepared by anodic arc plasma
WEI Zhi-qiang(魏智强)1, 2, XIA Tian-dong(夏天东)2, MA Jun (马 军)1, DAI Jian-feng(戴剑锋)1,
FENG Wang-jun(冯旺军)1, WANG Qing (王 青)1, YAN Peng-xun(闫鹏勋)3
1. School of Science, Lanzhou University of Technology, Lanzhou 730050, China;
2. College of Materials Science and Engineering, Lanzhou University of Technology, Lanzhou 730050, China;
3. Research Institute of Plasma and Metal Materials, Lanzhou University, Lanzhou 730000, China
Received 26 April 2005; accepted 15 August 2005
Abstract:
Based on the thermodynamics and kinetics theory, a theoretical model was built to illuminate the formation of metal nanopowders by anodic arc discharging plasma method, and the mechanism of particle nucleation and growth was investigated. In addition, the morphology, crystal structure, particle size and specific surface area of the nanopowders were characterized by X-ray diffraction(XRD), Brunauer-Emmett-Teller(BET) adsorption, transmission electron microscopy(TEM) and the corresponding selected area electron diffraction(SAED). The experimental results indicate that the nanopowders prepared by this process have uniform size, high purity, single phase and spherical shape. The crystal structure is FCC structure, the same as that of the bulk materials; the specific surface area is 12 m2/g, the particle size distribution ranges from 30 to 90 nm with an average particle size of 67 nm which is obtained from TEM and confirmed from XRD and BET results.
Key words:
anodic arc plasma; Cu nanopowders; nucleation; grain growth;
1 Introduction
In recent years, the research and development for metal nanopowders has attracted significant interest and it is still the subject of intense investigation owing to their intriguing properties and extensive prospects [1-5]. Metal nanopowders are used as conductive materials, catalysts, activation and sintering materials, high-dense magnetic recording materials etc[6-9]. All these applications require the powders consisting of monodisperse particles. The particle size and the structure strongly depend on the technique of the production. Many techniques have been developed to prepare metal nanopowders, such as chemical vapor deposition[10], spray pyrolysis[11], hydrothermal reaction[12], laser ablation[13], flame processing[14], vapor deposition[15], microwave-induced plasma synthesis[16], sol-gel method[17], and mechanical milling[18]. Among these methods, arc discharging plasma technique has attracted great interest because it is a promising route to prepare high quality nanopowders in large scale at low cost[19, 20]. However, the mechanism of nanoparticle nucleation and growth by this process has rarely been reported in literature. It is important for various materials and processes because these problems inherent in fully characterizing nanopowders microstructures and their evolution, especially it offers the possibility for obtaining nanopowders with desired physical and chemical properties.
In this paper, the formation of metal nanopowders by anodic arc discharging plasma technique was investigated, based on thermodynamics and kinetics theory a theoretical model was built to illuminate the mechanism of raw substance vapourisation, nucleation and grain growth, and the forming of nanoparticles. In addition, the samples were characterized by X-ray diffraction(XRD), Brunauer-Emmett-Teller(BET) adsorption, transmi- ssion electron microscopy(TEM) and the corres- ponding selected area electron diffraction(SAED).
2 Experimental
The metal nanopowders were prepared by anodic arc discharging plasma technique in inert atmosphere with homemade experimental apparatus which was described elsewhere[20]. The apparatus mainly consisted of the stainless steel vacuum chamber, the gas supply device, the DC power supply, the plasma generator with a high frequency initiator,the vacuum pump, the water-cooled collection cylinder, and the water-cooled copper crucible mounted in an electrically insulated manner and was connected to the arc current supply as anode, and the tungsten rod mounted in an insulated and axial sliding manner and connected to a power supply as cathode. The bulk raw material to be evaporated was placed in the crucible.
In the process of preparation, the vacuum chamber was pumped to 10-3 Pa and then was backfilled with inert argon (purity 99.99%) to near 103 Pa. The electric arc in the argon environment was automatically ignited between the wolfram electrode and the nozzle (well cooled) by high frequency initiator. Under argon pressure and electric discharge current, the ionized gases were driven through the nozzle outlet and formed the plasma jet[21]. The bulk metal Cu was heated and melted by the high temperature of the plasma, metal atom was detached from the metal surface when the plasma jet kinetic energy exceeded the metal superficial energy, and evaporated into free atom state. Above the evaporation source there was a region filled with supersaturated metal vapor, where the metal atoms diffused around and collided each other to decrease the nuclei forming energy. When the metal vapor was supersaturated, a new phase was nucleated homogeneously out of the aerosol systems[22]. The droplets were rapidly cooled and combined to form primary particles by an aggregation growth mechanism[23, 24]. The free inert gas convection developing between the hot evaporation source and the cooled collection cylinder transported the particles out of this nucleation and growth region to the inner walls of the cylinder. The loose nanopowders could be obtained after a period of passivation and stabilization with working gas.
The crystal structure of Cu nanopowders was analyzed by Japan Rigaku D/max-2400 X-ray diffractometer using monochromatic high-intensity Cu Ka radiation (l=1.540 56 ?, 40 kV, 100 mA). The average grain size of the particles was estimated from X-ray line broadening measurements according to Scherrer formula. The particle size and morphology shape were investigated by transmission electron microscopy(TEM) and the corresponding selected area electron diffraction(SAED) with a Japan JEOL JEM-1200EX microscope operating at an accelerating voltage of 80 kV. The specific surface area was measured by nitrogen sorption isotherms at 77 K. The data were conducted automatically on a micromeritics ASAP-2010 porosity analyzer (Micromerities Corp., U.S.A.). From the sorption data, the specific surface area of Cu nanopowders was evaluated by using the Brunauer- Emmett-Teller(BET) equation.
3 Results and discussion
3.1 Thermodynamics and kinetics for particle nucleation
Homogeneous nucleation process occurs in the absence of a solid interface, which plays an important role in controlling the particle size distribution. Thermodynamically, phase transformation can occur only when the free energy of the system decreases and cannot occur when the free energy of the system increases. The relative magnitude of the driving forces for the possible phase transformations dominates which phase is more likely to form under certain conditions. The metastable vapor phase has a higher free energy than the stable phase, and the thermodynamic quantities that dominate the nucleation process are the driving force for nucleation[23]. For supersaturated metal vapor, metal vapor atoms coalescence to produce embryos, the overall free energy change of the system is the sum of the free energy due to the formation of a new volume and the free energy due to the new surface created. For spherical particles, the difference in free energy can be calculated by
ΔG=(4/3)πr3Δg+4πr2σ (1)
where r is the radius of the embryos, σ is the surface free energy between the liquid and its vapor phase per unit surface area, Δg=-kT1nS is the volume free energy between the solid and its vapor phase per unit atom, k is the Boltzmann constant, T is the absolute temperature, and S= p/p0=exp(2σVm/rkT) is the super- saturation ratio, Vm is the molecular volume, p and p0 respectively stand for the vapor pressure and balance pressure on micron-nuclei surface.
When the saturation ratio S>1, ΔG has a positive maximum at a critical size. This maximum free energy is the activation energy for nucleation, embryos larger than the critical size will further decrease their free energy by growth and form stable nuclei that grow to form particles. The critical crystal nuclear size r* is expressed as
r*=![]() (2)
(2)
Eqn.(2) can be obtained by setting dΔG/dr=0, from which larger p/p0 helps to reduce the radius of critical crystal nuclei. At the same time, larger p/p0 helps to increase nanopowders productivity.
In this narrow region the further growth of the particles occurs mainly by coalescence to form large particles. For a given value of S, when the metal drops size is smaller than the critical nuclear size, namely, r>r*, nucleation stops and particles continue to grow until the critical nuclear size reaches. And all particles with r<r* will dissolve.
Hence the synthesis process can be expressed as the volume nucleation frequency at a temperature given by the classical homogeneous nucleation theory:
Cu(gas)→Cu(crystal nuclei)→Cu(nanopowders) (3)
Based on the nucleation thermodynamics, the particle formation depends on the ratio between the nucleation and the nuclei growth, the nucleation velocity In can be written as
In=A exp[![]() ] (4)
] (4)
where A is a kinetic parameter. Obviously, a larger p/p0 is beneficial to nucleation. It can be seen that a larger p/p0 value results in higher In at the initial period, while the reduction in p/p0 may lead to a decrease in In and an increase in growth velocity. Therefore the particles should be removed from growth zone as quickly as possible to restrict particle growth.
It should be pointed out that the initial particles in synthesis process are several nanometers and they are often in suspended state. Therefore, Brown motion law can describe their motions at this moment. Obviously, the interactions among particles will result in particle condensation. According to gas-molecular kinetics, the collision frequency can be written as
f=4(![]() kT/m)1/2dN2 (5)
kT/m)1/2dN2 (5)
where N is the particle concentration, m is the particle mass, d is the particle diameter, and k is the Boltzmann constant. The retention time of particles must be controlled effectively and the initial concentration of particles must be reduced in order to limit particle growth due to collision and agglomeration.
3. 2 Growth and aggregation after nucleation
In evaporation-condensation process, nanoparticles form from a supersaturated vapor in a narrow zone above the evaporation source by homogeneous nucleation followed by growth via condensation and coagulation. The nanoparticle formation and growth can be described as a Brown coagulation[24]. Each act of interaction between two particles leads to an instantaneous grain coalescence and the consequent formation of highly elongated particles. The precursors of the particles are nucleus. One single nucleus in vapor growth is generally relevant to the growth conditions and its environments.
A schematic representation of the formation mechanism for nanoparticles by nucleation and aggregation growth is shown in Fig.1. The synthesis system consists of metal evaporing zone, nucleation zone and growth zone. At the early stage of aging, as the progressing of raw substance evaporations in the metal evaporing zone, homogeneous nucleation occurs in the nucleation zone when the metal vapor is supersaturated. As the temperature increases, more and more nuclei are formed, some of them are rapidly cooled and combined to form primary particles by an aggregation growth mechanism. At the final stage of aging, a large number of nanoparticles are formed in the growth zone. Under the condition of sharp-cooling, particles are cooled down rapidly, the particle growth can be confined without affecting the nucleation rate. The free inert gas convection developing between the hot evaporation source and the cooled collection cylinder and travelling in the direction of the temperature gradient, transports the particles out of this nucleation and growth region to the cold collection zone, thus the growth process ends.

Fig.1 Schematic diagram illustrating growth process of nanoparticlers
In the growth process, besides the original nuclei by adsorption growth mode that particle nuclei grow with adsorption of metal atoms, the particle size can also be increased by coalescence growth mode that particles grow by collision with particle nuclei and clusters. Brownian motion and attractive Van der Waals forces will rapidly decrease the number of particles in the suspension. By this theory particles will grow until they reach a stable size. At this point they will grow by combining with smaller unstable nuclei and not by collisions with other stable particles. This shows that particle aggregation plays a significant role in determining the particle size of the final product[23].
3.3 Characterization of nanopowders
A small amount of the powder was dispersed in isopropanol by ultrasonic, and then deposited on copper grids with holey carbon coated film. The sample was dried in a vacuum oven at ambient temperature before examining. The sample was scanned in all zones before the picture was taken. Fig.2(a) shows the representative transmission electron microscopy(TEM) micrograph of Cu nanopowders. It can be seen from the graph that all of the particles are spherical in shape, small particles aggregate into secondary particles because of their extremely small dimensions and high surface energy, most of the particles appear fairly uniform in size, with smooth surface.

Fig.2 TEM micrograph (a) and SAED pattern (b) of Cu nanopowders
Fig.2(b) shows the corresponding selected-area electron diffraction(SAED) pattern. It can be indexed to the reflection of FCC structure in crystallography. The crystal structure was also investigated by means of X-ray diffraction. Tropism of the particles at random and small particles cause the widening of diffraction rings that make up of many diffraction spots, which indicates that the nanoparticles are polycrystalline structure. Electron diffraction reveals that each particle is composed of many small crystal nuclei, which is another proof that the particles grow by an aggregation mode.
From the data obtained by TEM micrographs, the particle size histograms can be drawn and the mean size of the particles can be determined. Fig.3 shows the particle size distribution of Cu nanopowders. It can be seen that the particle sizes range from 30 to 90 nm, and the median diameter (taken as average particle diameter) is about 67 nm, which shows a relatively narrow size distribution.
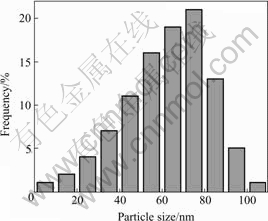
Fig.3 Particle size distribution of Cu nanopowders
Fig.4 shows the typical X-ray diffraction(XRD) pattern for the specimen. The relatively broad XRD peaks indicate the nano-crystalline nature of the particles. Five broad peaks with 2θ values of 43.44°, 50.56°, 74.24°, 90.04° and 95.24° correspond to (111), (200), (220), (311) and (222) planes of the bulk Cu respectively, which can be assigned to Cu face centered cubic(FCC) phase. The XRD spectrum does not reveal any other phase except the characteristic peaks of copper. This result shows that the physical phases of copper nanoparticles prepared in this work have high purity.

Fig.4 XRD pattern of Cu nanopowders
From the full width at half maximum, the average grain size can be estimated in conjunction with Scherrer equation: d=Kλ/Bcosθ, where d is the grain size, K= 0.89 is the Scherrer constant related to the shape and index (hkl) of the crystals, λ is the wavelength of the X-ray(Cu Kα, 0.149 54 nm), θ is the diffraction angle, and B is the corrected full width at half maximum. This result in an average grain size of 65 nm, which is consistent with the average particle diameter obtained from TEM image of Fig.2(a). This agreement indicates predominantly single crystalline primary particle.
The BET method based on low-temperature adsorption of nitrogen can be used to determine the specific surface area of the powder. If the powder consists of solid, spherical shape particles with smooth surface and same size, the relation between surface surface area and average equivalent particle size is equal to DBET =6 000/(ρ·Sw) (in nm), where DBET is the average diameter of a spherical particle, Sw represents the measured specific surface area of the powder in m2/g; and ρ is the particle density. Fig.5 shows the BET plots of Cu nanopowders. The specific surface area of the powders is 12 m2/g. The corresponding average equivalent particle size is 71 nm. In all cases, there is a good agreement between the average particle size obtained from TEM image and XRD pattern.

Fig.5 BET plot of Cu nanopowders
4 Conclusions
1) Copper nanopowders were successfully prepared by anodic arc discharging plasma method in inert atmosphere with homemade experimental apparatus. Nanoparticles formation from a supersaturated vapor by homogeneous nucleation followed the mechanism of adsorption growth and coalescence growth in the process.
2) The nanoparticles prepared by this process have uniform size, high purity, single phase and spherical shape. The crystalline structure of the particles is FCC structure, the same as that of the bulk copper; the spe-
cific surface area is 12 m2/g; the particle size distribution ranges from 30 to 90 nm with an average size of 67 nm.
References
[1] Gleiter H. Nanocrystalline materials[J]. Prog Mater Sci, 1990, 33(4): 223-315.
[2] Chen Y J, Cao M S, Tian Q. A novel preparation and surface decorated approach for a-Fe nanoparticles by chemical vapor-liquid reaction at low temperature[J]. Materials Letters, 2004, 58: 1481-1484.
[3] Zhang w w, Cao q q. Structural, morphological, and magnetic study of nanocrystalline cobalt-nickel-copper particles[J]. Journal of Colloid and Interface Science, 2003, 257: 237-243.
[4] Chen b j, Sun x w, Xu c x. Growth and characterization of zinc oxide nano/micro-fibers by thermal chemical reactions and vapor transport deposition in air[J]. Physica E, 2004, 21: 103-107.
[5] Chen d h, Chen d r. Hydrothermal synthesis and characterization of octahedral nickel ferrite particles[J]. Powder Technology, 2003, 133: 247-250.
[6] Cui z l, Dong l f, Hao c c. Microstructure and magnetic property of nano-Fe particles prepared by hydrogen arc plasma[J]. Mater Sci Eng, 2000, A286: 205-207.
[7] Lapatin v v, Ivanov u f, Dedkov v s. Structure-diffraction analysis of nanometer-sized polycrystals[J]. Nanostruct Mater, 1994, 4(6): 669-676.
[8] Ruslan z. Structure and mechanical properties of ultrafine-grained metals[J]. Mater Sci Eng, 1997, A234-236: 59-66.
[9] Gleiter h. Materials with ultrafine microstructures, retrospectives and perspectives[J]. Nanostruct Mater, 1992, 1 (1): 1-20.
[10] Cao m s, Deng q g. Synthesis of nitride-iron nano meter powder by thermal chemical vapor-phase reaction method[J]. Journal of Inorganic Chemistry, 1996, 12(1): 88-91.
[11] Karthikeyan j, Berndt c c, Tikkanen j. Plasma spray synthesis of nanomaterial powders and deposits[J]. Mater Sci Eng, 1997, A238: 275-286.
[12] Zheng h g, Lang j h, Zeng j h. Preparation of nickel nanopowders in ethanol-watersystem(EWS)[J]. Materials Research Bulletin, 2001, 36: 947-952.
[13] Gaertner g f, Miquel p f. Particle generation by laser ablation from solid targets in gas flows[J]. Nanostruct Mater, 1993, 4(3): 559-568.
[14] Katz j l, Miquel p f. Synthesis and applications of oxides and mixed oxides produced by a flame process[J]. Nanostruct Mater, 1994, 4(5): 551-557.
[15] Gunther b, Kumpmann a. Ultrafine oxide powders prepared by inert gas evaporation[J]. Nanostruct Mater, 1992, 1(1): 27-30.
[16] Vollath d, Sickafus k e. Synthesis of nanosized ceramic oxide powders by microwave plasma reactions[J]. Nanostruct Mater, 1992, 1(5): 427-437.
[17] Chen d h, He x r. Synthesis of nickel ferrite nanoparticles by sol-gel method[J]. Materials Research Bulletin, 2001, 36: 1369-1377.
[18] Koch c c. The synthesis and structure of nanocrystalline materials produced by mechanical attrition: a review[J]. Nanostruct Mater, 1993, 2(2): 109-129.
[19] IOAN B. Nanoparticle production by plasma[J]. Matel Sci Eng, 1999, B68: 5-9.
[20] Wei z q, Qiao h x, Yan p x. Preparation and characterization of Ni nanopowders prepared by anodic arc plasma[J]. Trans Nonferrous Met Soc China, 2005, 15(1): 51-56.
[21] Andre j, Kwan w. Arc-discharge ion sources for heavy ion fusion[J]. Nuclear Instruments and Methods in Physics Research, 2001, A464: 569-575.
[22] Scott j h, Majetich s a. Morphology, structure, and growth of nanoparticles produced in a carbon arc[J]. Phys Rev, 1995, B52: 12564-12571.
[23] Wangz l, Liu y, Zhang z. Handbook of Nanophase and Nanostructed Materials(Ⅲ)[M]. Beijing: Tsinghua University Press, 2002. 111-120.
[24] Kaito c. Coalescence growth mechanism of smoke particles[J]. Jpn J Appl Phys, 1985, 24:261-264.
Foundation item: Project (3ZS042-B25-017) supported by the Natural Science Foundation of Gansu Province, China
Corresponding author: WEI Zhi-qiang; Tel: +86-931-2975732; E-mail: zqwei7411@163.com
(Edited by YUAN Sai-qian)


