
Vacuum distillation refining of metallurgical grade silicon (Ⅱ)——Kinetics on removal of phosphorus from metallurgical grade silicon
MA Wen-hui(马文会)1, 2, WEI Kui-xian(魏奎先)1, 2, YANG Bin(杨 斌)1, 2,
LIU Da-chun(刘大春)1, 2, DAI Yong-nian(戴永年)1, 2
1. National Engineering Laboratory for Vacuum Metallurgy, Faculty for Materials and Metallurgical Engineering, Kunming University of Science and Technology, Kunming 650093, China;
2. Key Laboratory of Vacuum Metallurgy of Non-ferrous Metals of Yunnan Province, Kunming 650093, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
The kinetics on vacuum refining process of metallurgical grade silicon was studied using maximum evaporation rate, critical pressure and mean free path of phosphorus in the metallurgical grade silicon at different temperatures. The behaviors of impurity phosphorus in the vacuum distillation process were examined in detail. The results show that the fractional vacuum distillation should be taken to obtain silicon with high purity. Impurity phosphorus volatilize with the maximum evaporation rate of 1.150×105- 1.585×106 g/(cm2·min) in the temperature range of 1 073-2 173 K and the pressure below 2.1 Pa. Because the value of ωmax, P is at least 108 times of ωmax, Si, Si hardly evaporates and remains in the residual, which indicates that phosphorus can be removed from metallurgical grade silicon (MG-Si) completely.
Key words:
metallurgical grade silicon; solar grade silicon; vacuum distillation; kinetics; phosphorus removal;
1 IntroductionSince solar cell was invented in Bell-lab, silicon was in predominant position as raw materials[1]. With the fast development of photovoltaic industry and the gradual resuscitation of semiconductor industry[2], these scraps came primarily from semiconductor industry have not meet the rapid development of solar energy industry [3]. Silicon material is one of the primary bottle-necks which impede the development of photovoltaic industry [4-5]. The metallurgical grade silicon (MG-Si) was obtained from the reduction of silica in a furnace by carbon[6]. However, MG-Si contains some impurities including Fe, Al, Ca and P and so on, which affect the physiochemical properties of silicon materials. Phosphorus is one of the impurities that are difficult to remove, and the required maximum limit for phosphorus content is 0.1×10-6 for solar grade silicon (SOG-Si)[7]. It is of great importance to carry out a fundamental study for solar grade silicon production. Thermodynamics on separating impurity phosphorus from the metallurgical grade silicon by vacuum distillation was studied in our previous work[8], which solves some problems, such as the separation possibility of impurity phosphorus from metallurgical grade silicon by vacuum distillation, the separation extent and the P content of distilled metallurgical grade silicon. However, the production efficiency, namely the evaporation rate, is more important in practice. Therefore, it is necessary to study the kinetics in the vacuum distillation process of metallurgical grade silicon. It has proved that the evaporation rate of element is affected mainly by temperature and pressure. This study is aimed at the influence of temperature, pressure and free mean path on the evaporation rate of phosphorus in metallurgical grade silicon.
2 Relationship between maximum evapora- tion rate and temperature
When the total chamber pressure (p) is 1ess than the saturation vapor pressure of a pure element ![]() and the mean free path (l) of the vaporized molecule is greater than the effective dimension (l, the distance between evaporating and condensing surface) of the unit, the evaporation rate of the distilled element is maximum, which can be described as
and the mean free path (l) of the vaporized molecule is greater than the effective dimension (l, the distance between evaporating and condensing surface) of the unit, the evaporation rate of the distilled element is maximum, which can be described as
![]() (1)
(1)
where wmax is the maximum evaporation rate, g/(cm2·min); a is the distillation coefficient; M is the relative molecular mass of the distilled element, ![]() is the saturation vapor pressure of the distilled element, Pa; and T is the melt surface temperature, K, respectively.
is the saturation vapor pressure of the distilled element, Pa; and T is the melt surface temperature, K, respectively.
The relationship between saturation vapor pressure of pure elements (![]() Pa) in the metallurgical grade silicon and temperature (T, K) is listed as follows[9-10]
Pa) in the metallurgical grade silicon and temperature (T, K) is listed as follows[9-10]
![]() (2)
(2)
![]() (3)
(3)
Supposing that the distillation coefficient αi is 1, the maximum evaporation rate of each element contained in the metallurgical grade silicon is calculated in the temperature range of 1 073-2 173 K according to Eqns. (2) and (3), as presented in Table 1.
Table 1 Maximum evaporation rates ωmax of phosphorus and silicon at different temperatures
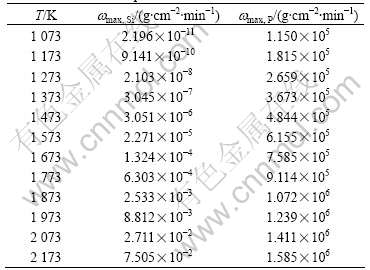
It can be seen from Table 1 that the maximum evaporation rate is affected remarkably by the distillation temperature. The vaporation rate can be enhanced by several or even tens orders of magnitude with the temperature increment of 100 K. It is concluded that phosphorus is volatilized quickly and concentrated into vapor phase. It is easy to separate impurity phosphorus from the metallurgical grade silicon completely because the value of wmax, P is 108-1010 times greater than that of wmax, Si in the temperature range of 1 573-2 173 K. Therefore, vacuum distillation can be taken to obtain silicon with lower content of P. The result is consistent with that of the thermodynamic discussion [8].
3 Influence of pressure on evaporation rateThe evaporation of metals or alloys can be promoted at vacuum atmosphere. Many studies[11-13] have shown that the evaporation rate (w) increased with the decrease of chamber pressure ( p) in a wide range ( p>pcrit, as shown in Fig.1). When p reduces to a specific value or below, the w does not increase any more or just increases at an ignorable amount. This specific value is called as critical pressure pcrit, which is great helpful for the selection of optimized pressure. The critical pressure means that w is the wmax in practice. It rises with enhancing temperature.
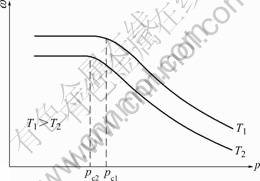
Fig.1 Relationship between evaporation rate and chamber pressure
The critical pressure is determined by a useful scattering cross-section of vapor molecular and its mass as[10]
 (4)
(4)
![]() (5)
(5)
where Mm is the relative molecular mass of the metal vapor, Mg is the relative molecular mass of the residual gas vapor, dm is the diameter of the metal vapor molecular, dg is the diameter of the residual gas vapor molecular, σ is the mean useful cross-section of the vapor molecular, k is Boltzmann constant (k=1.38×10-23 J/K), and l is the mean free path, respectively.
During the experiment, we used argon as the protective gas and the distance between evaporating and condensing surface is 15 cm in vacuum distillation for MG-Si refining. It is considered that argon is the residual gas and l is 15 cm. Thus, the critical pressures of the phosphorus in the MG-Si calculated by Eqns.(4) and (5) on basis of the values of Mm, Mg, dm and dg are listed in Table 2.
Table 2 Relationship between critical pressure pcrit of pure metal and temperature
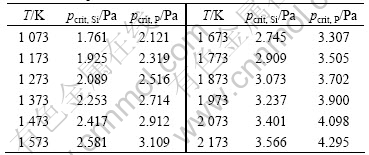
It is shown from Table 2 that the critical pressure is related to the evaporation temperature, and pcrit enhances with the increase in temperature. The value of pcrit, Si is 1.761-3.566 Pa in the temperature range of 1 073-2 173 K, and the value of pcrit, P is 2.121-4.295 Pa in the temperature range of 1 073-2 173 K. The minimum chamber pressure should be less than 2.1 Pa to ensure the maximum evaporation rate of P reach the range of 1.150×105-1.585×106 g/(cm2·min) while the silicon volatilizes at lower rate far less than the wmax,P. Because the value of wmax,P is at least 108 times of wmax,Si, Si hardly evaporates and remains in the residual. Phosphorus could be separated from MG-Si completely.
4 Effect of temperature and pressure on mean free pathAs mentioned above, the mean free path (l) of the distilled vapor molecule is greater than or equals to the effective dimension (l) of the chamber, under which the evaporation rate is not affected by chamber pressure any more. When the vapor molecular of distilled silicon is considered as monatomic one, the effect of temperature and pressure on mean free path can be described as[14]
![]() (6)
(6)
where p is the chamber pressure, Pa; and d is the diameter of phosphorus vapor molecular, dP=2.46×10-8 cm.
According to Eqn.(6), the mean free path values of distilled phosphorus vapor molecular are calculated in the temperature range of 1 073-2 173 K and pressure 0.00l-10 Pa, as shown in Fig.2.
It can be seen from Fig.2 that the minimum distillation pressure can be controlled in 0.5-1.0 Pa for matching l at the temperature of 1 773 K. The minimum pressure is considered the critical pressure of the condition above. Phosphorus evaporates quickly at the maximum rate of 1.150×105-1.585×106 g/(cm2·min). The evaporation rate does not increase with lowering distillation pressure. Also from Fig.2, l increases with enhancing temperature, which indicates that pcrit behaves in the same way.
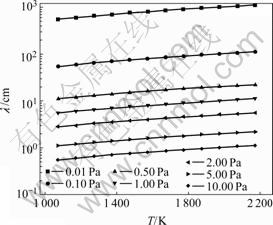
Fig.2 Mean free path of distilled phosphorus vapor molecular
Therefore, the evaporation rate of metal relates to distillation temperature as well as the value of residual pressure. It is necessary to control the elevated temperature and low chamber pressure for separating impurity phosphorus efficiently from the silicon in the process of MG-Si refining.
5 Conclusions1) It is an effective route to remove phouphorus using vacuum distillation for MG-Si refining.
2) Impurity phosphorus volatilizes at the maximum evaporation rate of 1.150×105-1.585×106 g/(cm2·min) in the temperature range of 1 073-2 173 K and the pressure below 2.1 Pa.
3) Because the value of wmax, P is at least 108 times of ωmax,Si, Si hardly evaporates and remains in the residual. Phosphorus can be separated from MG-Si completely.
References[1] GOETZBERGER A, HEBLING C. Photovoltaic materials, past, present, future [J]. Solar Energy Materials & Solar Cells, 2000, 62: 1-19.
[2] KHATTAK C P, JOYCE D B, SCJMID F. A simple process to remove boron from metallurgical grade silicon [J]. Solar Energy Materials & Solar Cells, 2002, 74: 77-89.
[3] MA Wen-hui, OGURA M, KOBAYASHI T, TAKAHASHI H. Preparation of solar grade silicon from optical fibers wastes with thermal plasmas [J]. Solar Energy Materials & Solar Cells, 2004, 81: 477-483.
[4] WODITSCH P, KOCH W. Solar grade silicon feedstock supply for PV Industry [J]. Solar Energy Materials & Solar Cells, 2002, 72: 11-26.
[5] SARTI D, EINHAUS R. Silicon feedstock for multi-crystalline photovoltaic industry [J]. Solar Energy Materials & Solar Cells, 2002, 72: 27-40.
[6] PIRES J C S, BRAGA A F B, MEI P R. Profile of impurities in polycrystalline silicon samples purified in an electron beam melting furnace [J]. Solar Energy Materials & Solar Cells, 2003, 79: 347-355.
[7] DAVIS J R, ROHATGI A Jr, HOPKINS R H, BLAISP D, RAI-CHOUDHURY P, McCORMICK J R, MOLLENKOPF H C. Impurities in silicon solar cells [J]. Electron Devices, IEEE Transactions on Electron Devices, 1980, 27: 677-687.
[8] WEI Kui-xian, MA Wen-hui, DAI Yong-nian, YANG Bin, LIU Da-chun, WANG Jing-fu. Vacuum distillation refining of metallurgical grade silicon (I): Thermodynamics on removal phosphorus from metallurgical grade silicon [J]. Trans of Nonferrous Met Soc of China, 2007, s1: 1022-1025.
[9] KUBASCHEWSKI O, ALCOCK C B. Metallurgical Thermochemistry [M]. Beijing: Metallurgical Industry Press, 1985: 486-513. (in Chinese)
[10] DAI Yong-nian, ZHAO Zhong. Vacuum metallurgy [M]. Beijing: Metallurgical Industry Press, 1988: 114-115. (in Chinese)
[11] DAI Yong-nian, XIA Dan-kui, CHEN Yan, CAI Xiao-lan, YANG Bin, LI Jin-hua, DENG Zhi-ming, WEI Zong-ping. Evaporation of metals in vacuum [J]. Journal of Kunming Institute of Technology, 1994, 19(6): 26-32. (in Chinese)
[12] DAI Yong-nian, ZHANG Guo-jing. The separation of Pb-Sb alloys by vacuum distillation [J]. Trans Nonferrous Met Soc China, 1991, 1(1): 44-49, 60.
[13] YANG Bin, DAI Yong-nian, ZHANG Guo-jing, WU Kun-hua. The separation of Sn-Sb alloys by vacuum distillation [J]. Journal of Kunming University of Science and Technology, l998, 23(3): 104-107. (in Chinese)
[14] LIU Yi-1un. Basic chemistry of elements [M]. Beijing: Higher Education Press, 1992: 173-506. (in Chinese)
Foundation items: Project(50674050) supported by the National Natural Science Foundation of China; Project(2006BAE01B08) supported by the Sustentation Project of Science and Technology of China; Project(20060674004) supported by Doctorial Programs Foundation of Ministry of Education of China.
Corresponding author: MA Wen-hui; Tel: +86-871-5161583; E-mail: mwhui@kmust.edu.cn


