Trans. Nonferrous Met. Soc. China 25(2015) 199-205
Properties of Zn-Bi composite coatings prepared by ionic co-discharge deposition
See Leng TAY1,2, Wei-wei CHEN1,3, Xiao-jin WEI1, Cai-zhen YAO1, Wei GAO1
1. Department of Chemical and Materials Engineering, the University of Auckland, Auckland 1142, New Zealand;
2. Department of Mechanical Engineering Department, University of Malaya, Kuala Lumpur 50603, Malaysia;
3. Department of Materials Science and Technology, Beijing Institute of Technology, Beijing 100081, China
Received 21 February 2014; accepted 1 July 2014
Abstract:
Zn-Bi composite was synthesized by ionic co-discharge deposition and its properties were investigated. The results show that the Zn-Bi composite with the incorporation of Bi has a finer grain size than the pure Zn coating and improves the mechanical properties. The microhardness is increased by approximately two times simply by adding a small amount of Bi electrolyte into a Zn bath solution. A lower volume loss of the Zn-Bi composite coating compared with the pure Zn coating also indicates that the Zn-Bi coating has a better wear resistance.
Key words:
Zn-Bi composite coating; ionic co-discharge deposition; electroplating; mechanical properties;
1 Introduction
Zn coatings are the most commonly used non-noble metal coatings to protect the substrate by cathodic control. About half of the product Zn in the world is used for protection of corrosion. It is widely used because of its relatively low price, adequate supply, flexibility in application, ease to control the thickness of coating, good cathodic protection to steel, and the ability of combining with other elements to form special alloy coatings for special properties [1,2].
Composite coatings contain a dispersion of second phase, usually in the form of particles, to improve material properties such as hardness, wear resistance, self-lubrication, and corrosion resistance [3-5]. Various types of Zn composite coatings such as Zn-Al2O3 [6], Zn-MoS2 [7], Zn-TiO2 [8], and Zn-yttria stabilized zirconia (YSZ) [9] have been studied recently. Researchers found out that the Zn composite coatings enhanced mechanical properties and showed better sacrificial protection ability on steels than the pure Zn coating [8].
There are a number of methods to produce metal matrix composites in electroplating. One of the conventional ways is adding solid particles into the bath solution with vigorous agitation to form a homogenous distribution of fine-particles. It is an important factor in determining the properties of composite coatings [10].
A novel method called an ionic co-discharge process is introduced to obtain a homogenous dispersion. This method is to have different ions in the electrolyte and allow them to discharge to the cathode at the same time. If the metals do not dissolve each other or form intermetallic compounds, a two or more phases composite coating will be formed. The details were reported in our previous study [11].
In the present study, Zn-Bi composite coatings were developed, and their phase structure, microstructure, microhardness and wear property were characterized. Zn and Bi are two insoluble metals in their solid state; the two-phase composite is therefore expected to form.
2 Experimental
Mild carbon steel plate with the dimensions of 15 mm×25 mm was used as substrate. Before electroplating, the substrate was ground with the sand paper to a grit of 1200, and then degreased in ethanol. The pre-treatment of the substrate also included activation by acid pickling with 1 mol/L HCl for 60 s. The electrolyte solution and electroplating parameters are listed in Table 1.
Table 1 Composition of electroplating bath and processing parameters
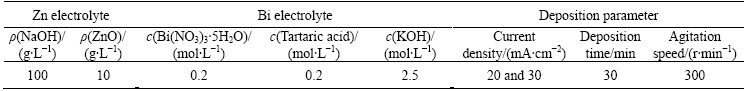
The Zn and Bi bath solution was prepared separately. 1 mL of 0.2 mol/L Bi electroplating solution was added into 70 mL of Zn electrolyte to make Zn-Bi composite. The pure Zn coating was also electroplated for comparison.
The morphology and Bi concentration in the Zn-Bi coatings were measured by a scanning electron microscope (SEM) with an energy dispersion spectroscopy (EDS) attachment. The phase structure of the coatings was determined by X-ray diffraction (XRD). The coating hardness was measured by a microhardness tester (Leco M400) with a Vickers diamond indenter. The applied load was 50 g with a holding time of 10 s. At least 5 measurements under the same conditions were conducted, and the average value was used as the microhardness (HV). The standard deviation was also calculated.
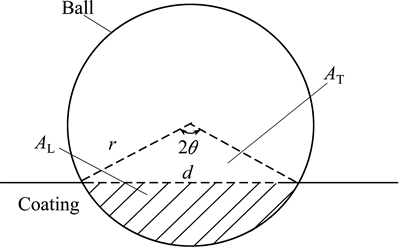
Fig. 1 Diagram for derivation of wear volume loss in wear testing
The wear property of the coatings was tested by NANOVEA Tribometer where a ceramic ball of 6 mm in diameter acted as friction counterpart. The test was conducted with a load of 1 N and sliding speed of 100 r/min at room temperature with the relative humidity of ~50%. The wear volume was calculated based on Fig. 1.
 ,
, 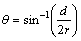 .
.
The area is subtended by 2θ, 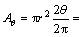
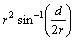 .
.
Area of the triangle 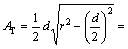
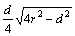 .
.
The shaded worn away area is
 (1)
(1)
Hence, the wear volume loss is
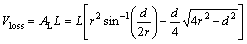 (2)
(2)
where L is the length of the wear track; r is the radius of the ball used for wear testing (6 mm); d is the wear track width.
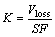 (3)
(3)
where K is wear rate; S is the sliding distance; F is the applied load.
The wear track was measured with the optical microscope (Olympus BX60M), and the wear volume loss and wear rate were also calculated by Eqs. (2) and (3), respectively.
3 Results and discussion
3.1 Phase structure and orientation
Figure 2 shows the XRD patterns of Zn and Zn-Bi coatings deposited at 20 and 30 mA/cm2 for 30 min. The two main peaks of the Zn coatings were Zn (101) and Zn (100). There were no different phases in the Zn or Zn-Bi coatings with different current densities. However, co-deposition of Bi into Zn matrix showed the Bi phase and changed the grain texture. Upon incorporation with Bi, the Zn (101) peak was decreased at both current densities. However, Zn (002) was slightly enhanced under the current density of 30 mA/cm2.
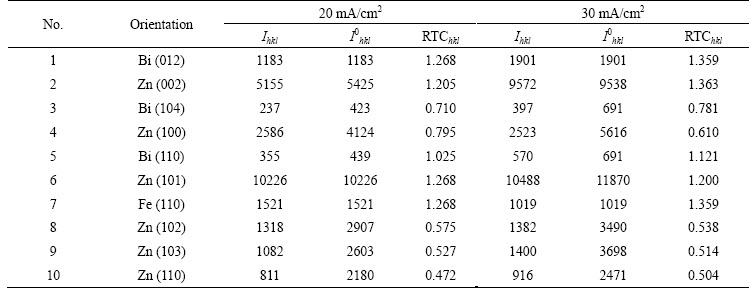
The relative texture coefficients of Zn and Zn-Bi electrodeposition are shown in Tables 2 and 3, respectively. The relative texture coefficient (RTC) of the Zn coatings was calculated using the following equation [12,13]:
where Ihkl and 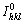 are the diffraction intensities of the hkl plane measured in the diffractogram of the deposit and the standard Zn powder sample. SEN et al [14] reported that a plane was namely as preferred orientation if its texture coefficient was greater than 1.0. Based on this calculation, two similar preferred crystal orientations of Zn (100) and Zn (101) occurred at different current densities. Upon addition of Bi, the preferred crystal orientations changed to Zn (002) and Zn (101). A similar trend has also been reported with the incorporation of MoS2 particles into Zn electrolyte where the preferred crystal orientation changed from Zn (002) to Zn (101) and Zn (112) [7].
are the diffraction intensities of the hkl plane measured in the diffractogram of the deposit and the standard Zn powder sample. SEN et al [14] reported that a plane was namely as preferred orientation if its texture coefficient was greater than 1.0. Based on this calculation, two similar preferred crystal orientations of Zn (100) and Zn (101) occurred at different current densities. Upon addition of Bi, the preferred crystal orientations changed to Zn (002) and Zn (101). A similar trend has also been reported with the incorporation of MoS2 particles into Zn electrolyte where the preferred crystal orientation changed from Zn (002) to Zn (101) and Zn (112) [7].
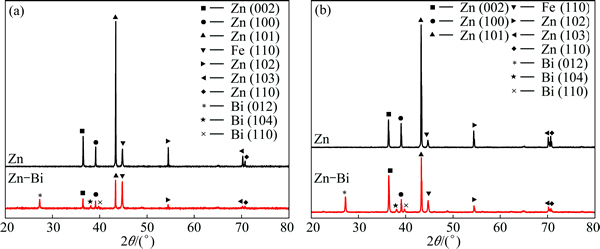
Fig. 2 XRD patterns of Zn and Zn-Bi composite coatings deposited at 20 mA/cm2 (a) and 30 mA/cm2 (b)
Table 2 Relative texture coefficient (RTC) of Zn coatings

Table 3 Relative texture coefficient (RTC) of Zn-Bi coatings
3.2 Microstructure characterization
Figure 3 depicts the top-view morphology of Zn and Zn-Bi coatings. The irregular microstructure shape was observed on the pure Zn coating. In the presence of Bi, a finer and round structure formed in the Zn-Bi composite coating. This might be due to the fact that Bi provided more nucleation sites and inhibited the growth of Zn metal. Hence, Zn-Bi composite coating showed a fine- grained structure. Similar results were also reported in the case of incorporation TiO2 [15,16], or MoS2 [7] into Zn electrodeposition.
The cross-sectional microstructures of Zn and Zn-Bi coatings are shown in Fig. 4. A thicker coating was deposited in the composite coating compared with the pure Zn electrodeposition. The thicknesses of Zn-Bi composite coating deposited at 20 and 30 mA/cm2 were (10.6±0.4) and (16.9±0.9) μm, and (7.5±0.7) and (14.1±1.4) μm for Zn coating, respectively, indicating that Bi ions enhanced the deposition rate.
3.3 Chemical analysis
Table 4 shows EDS analysis of the Bi content electrodeposited in the Zn coating at different current densities for the same deposition time of 30 min. The Bi content in the coating increased with the current density. This implies that the higher current density would promote more Bi ions deposition. SHI et al [17] also reported that the content of codeposition of SiC in the Ni-Co coating was increased with increasing the current density up to 20 mA/cm2. By increasing the current density, there was a tendency for more particles to deposit on the substrate.
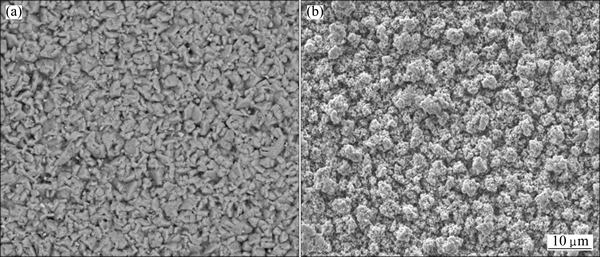
Fig. 3 Top surface morphologies of Zn coating (a) and Zn-Bi coating (b) deposited at 30 mA/cm2
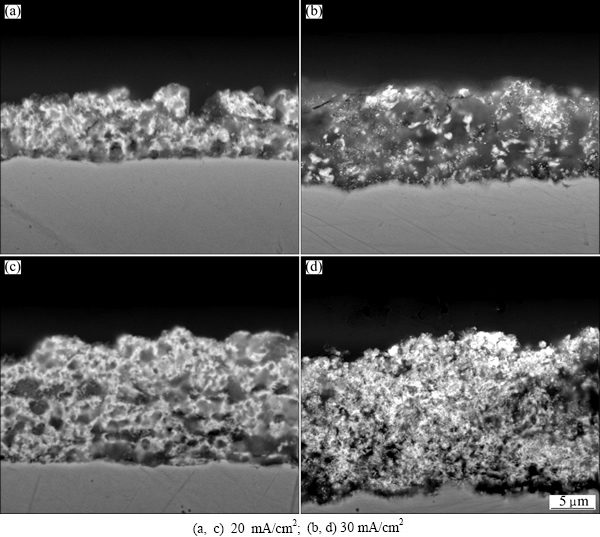
Fig. 4 Cross-sectional morphologies of Zn (a, b) and Zn-Bi (c, d) coatings at different current densities
Table 4 EDS results of Bi content electrodeposited in Zn-Bi coating at different current densities

3.4 Mechanical properties
3.4.1 Microhardness
The microhardness of the coatings deposited at two current densities is shown in Fig. 5, showing a significant improvement of hardness by addition of Bi. The average microhardness of Zn coating deposited at the 20 and 30 mA/cm2 are HV 102 and HV 108, respectively. With Bi addition, the microhardnesses increase to HV 183 and HV 200, respectively. The hardness is increased by almost 2 times with just the addition of 1 mL Bi electrolyte into 70 mL of Zn bath solution.
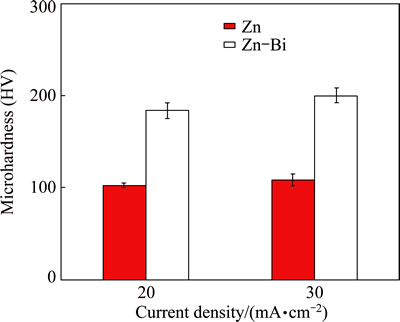
Fig. 5 Microhardness of Zn and Zn-Bi coatings deposited at different current densities
In comparison, only a small increase of microhardness at the higher current density for the Zn-Bi composite coatings is found, even though the Bi contents are quite different with different current densities. This implies that Bi content is not the only influential factor for the mechanical property. This result also shows the same trend with the addition of Bi into the Cu coating in our previous study [18].
It is known that several factors influence the mechanical properties of composite coatings, including dispersion strengthening due to the Orawan mechanism, and grain-refinement effect. The second phase of Bi is insoluble with the Zn metal, which is well dispersed in the Zn matrix and hinders the dislocation movement in the alloy [19]. The grain refinement strengthening mechanism follows the Hall-Petch relationship. As shown in Fig. 3, a fine grained structure formed with the addition of Bi into Zn coating. The higher density of grain boundaries could impede the movement of dislocation, and thus enhance the microhardness [16,20]. PARAVEEN and VENKATESHA [16] also reported that the addition of TiO2 into Zn coating enhanced the microhardness due to the fine grained structure of the deposit.
3.4.2 Wear testing
Figure 6 shows the volume loss and wear rate of the coatings deposited at different current densities. The volume loss or wear rate of the Zn-Bi composite coating is lower compared with that of the Zn coating, indicating that the Zn-Bi coating has a better wear resistance compared with the Zn coating.

Fig. 6 Volume loss and wear rate of Zn and Zn-Bi coatings at different current densities
Figure 7 represents the wear tracks of Zn and Zn-Bi composite coatings. The widths of wear tracks of Zn and Zn-Bi composite coatings were ~660 μm and 525 μm, respectively. Many plough lines were observed on the surface of the Zn coating. In contrast, the wear tracks on the Zn-Bi composite coatings were narrower and the plough lines were shallower, indicating that the Zn-Bi composite coatings had improved the wear resistance.
According to the Archard’s law [21], the volume loss during the sliding wear is proportional to the work done by the friction force and inversely to the hardness of the contact surface. Hence, the higher microhardness of the coating had a lower volume loss. The microhardness of the Zn-Bi coatings as indicated earlier, proved that the lower wear volume loss of composite coating came from the higher microhardness.
An opposite effect was found with the coating deposited at different current densities. At the higher current density of 30 mA/cm2, both Zn and Zn-Bi coatings showed a slightly higher microhardness than those deposited at 20 mA/cm2. However, the volume loss did not show improvement, implying that other factors such as microstructure may also play a role in the wear performance [22]. It is interesting to note that there are two opposing properties which are ductility and hardness that could influence the wear value [22]. A softer surface normally contains higher ductility and greater plastic deformation to reduce the wear volume loss. On the other hand, increasing the hardness enhances the wear resistance by retarding the dislocation motion. However, the increment of the hardness decreases the ductility of the material at the same time.
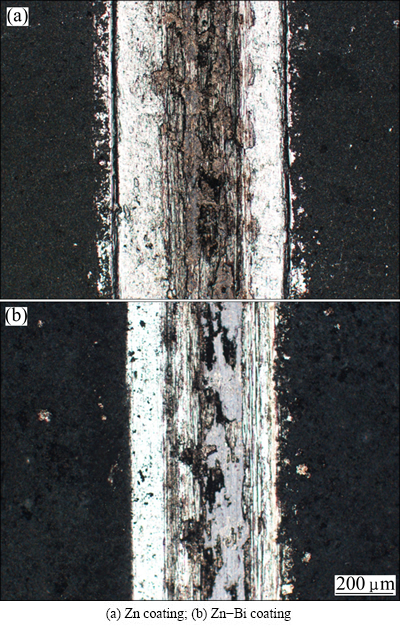
Fig. 7 Wear tracks on coatings deposited at 20 mA/cm2 for 30 min
4 Conclusions
The Zn-Bi composite coating was prepared by ionic co-discharge deposition. The coating possesses a finer grained microstructure with dispersive Bi phase. The Bi content could be adjusted by controlling the deposition current density and time. The mechanical properties including hardness and wear resistance are improved significantly. The incorporation of Bi also enhances the deposition rate.
Acknowledgements
The authors would like to thank the technical staff and research group members in the Department of Chemical and Materials Engineering, the University of Auckland, for their valuable assistance. See Leng TAY appreciates the financial support from the Bright Spark Unit, University of Malaya.
References
[1] SCHWEITZER P A. Coatings corrosion of linings & coatings: Cathodic and inhibitor protection and corrosion monitoring [M]. Florida, United States of America: CRC Press, 2007: 403-485.
[2] SCHWEITZER P A. Fundamentals of metallic corrosion: Atmospheric and media corrosion metals [M]. Florida, United States of America: CRC Press, 2007: 623-643.
[3] HOVESTAD A, JANSEEN L J J. Eletroplating of metal matrix composites by codeposition of suspended particles [M]//Modern aspects of electrochemistry. New York, Boston, Dordrecht, London, Moscow: Kluwer Academic Publishers, 2005: 475-532.
[4] COJOCARU P, SPREAFICO M, GOMEZ E,  E, MAGAGNIN L. Electrocodeposition of CoNi/barium ferrite using a forced flow cell [J]. Surface and Coatings Technology, 2010, 205(1): 195-199.
E, MAGAGNIN L. Electrocodeposition of CoNi/barium ferrite using a forced flow cell [J]. Surface and Coatings Technology, 2010, 205(1): 195-199.
[5] MOHAN S, SARAVANAN G, BUND A. Role of magnetic forces in pulse electrochemical deposition of Ni nano Al2O3 composites [J]. Electrochimica Acta, 2012, 64: 94-99.
[6] SANCAKOGLU O, CULHA O, TOPARLI M, AGADAY B, CELIK E. Co-deposited Zn-submicron sized Al2O3 composite coatings: Production, characterization and micromechanical properties [J]. Materials & Design, 2011, 32(7): 4054-4061.
[7] KANAGALASARA V, VENKATESHA T. Studies on electrodeposition of Zn–MoS2 nanocomposite coatings on mild steel and its properties [J]. Journal of Solid State Electrochemistry, 2012, 16(3): 993-1001.
[8] VLASA A, VARVARA S, POP A, BULEA C, MURESAN L M. Electrodeposited Zn-TiO2 nanocomposite coatings and their corrosion behavior [J]. Journal of Applied Electrochemistry, 2010, 40(8): 1519-1527.
[9] XIA X, ZHITOMIRSKY I, MCDERMID J R. Electrodeposition of zinc and composite zinc–yttria stabilized zirconia coatings [J]. Journal of Materials Processing Technology, 2009, 209(5): 2632-2640.
[10] CARLBERG T, FREDRIKSSON H. The influence of microgravity on the solidification of Zn-Bi immiscible alloys [J]. Metallurgical and Materials Transactions A, 1980, 11(10): 1665-1676.
[11] CHEN W, WANG L, GAO W. Synthesis of Zn-Bi nano-composite coatings by an ionic co-discharge process [J]. Chemical Engineering Journal, 2012, 192(1): 242-245.
[12] THIEMIG D, BUND A. Characterization of electrodeposited Ni-TiO2 nanocomposite coatings [J]. Surface and Coatings Technology, 2007, 202(13): 2976-2984.
[13] SPANOU S, PAVLATOU E A, SPYRELLIS N. Ni/nano-TiO2 composite electrodeposits: Textural and structural modifications [J]. Electrochimica Acta, 2009, 54(9): 2547-2555.
[14] SEN R, DAS S, DAS K. Influence of duty cycle on the microstructure and microhardness of pulse electrodeposited Ni-CeO2 nanocomposite coating [J]. Materials Research Bulletin, 2012, 47(2): 478-485.
[15] GOMES A, FRADE T, SILVA PEREIRA M I. Studies on the stability of Zn and Zn-TiO2 nanocomposite coatings prepared by pulse reverse current [J]. ECS Transactions, 2010, 28(24): 13-23.
[16] PARAVEEN B M, VENKATESHA T V. Electrodeposition and properties of Zn-nanosized TiO2 composite coatings [J]. Applied Surface Science, 2008, 254(8): 2418-2424.
[17] SHI L, SUN C, GAO P, ZHOU F, LIU W. Mechanical properties and wear and corrosion resistance of electrodeposited Ni-Co/SiC nanocomposite coating [J]. Applied Surface Science, 2006, 252(10): 3591-3599.
[18] WEI Xiao-jin, CHEN Wei-wei, WANG Yu-xin, TAY See-Leng, GAO Wei. Microstructures and mechanical properties of electroplated Cu-Bi coatings [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(10): 2939-2944.
[19]  UYSAL M, ASLAN S, ALP A, AKBULUT H. Effect of particle concentration on the structure and tribological properties of submicron particle SiC reinforced Ni metal matrix composite (MMC) coatings produced by electrodeposition [J]. Applied Surface Science, 2012, 258(10): 4260-4267.
UYSAL M, ASLAN S, ALP A, AKBULUT H. Effect of particle concentration on the structure and tribological properties of submicron particle SiC reinforced Ni metal matrix composite (MMC) coatings produced by electrodeposition [J]. Applied Surface Science, 2012, 258(10): 4260-4267.
[20]  ALP A, AKBULUT H. Characteristics of electro-co-deposited Ni-Al2O3 nano-particle reinforced metal matrix composite (MMC) coatings [J]. Wear, 2009, 267(5-8): 976-990.
ALP A, AKBULUT H. Characteristics of electro-co-deposited Ni-Al2O3 nano-particle reinforced metal matrix composite (MMC) coatings [J]. Wear, 2009, 267(5-8): 976-990.
[21] ARCHARD J F. Contact and rubbing of flat surfaces [J]. Journal of Applied Physics, 1953, 24(8): 981-988.
[22] HOSSON J T M, CAVALEIRO A. Galileo comes to the surface![M]//Nanostructured coatings. New York: Springer, 2006: 1-26.
离子共放电沉积法制备Zn-Bi复合镀层及其性能
See Leng TAY 1,2,陈为为1,3,魏晓金1,姚彩珍1,高 唯1
1. Department of Chemical and Materials Engineering, the University of Auckland, Auckland 1142, New Zealand;
2. Department of Mechanical Engineering Department, University of Malaya, Kuala Lumpur 50603, Malaysia;
3. 北京理工大学 材料科学与工程系,北京 100081
摘 要:采用离子共放电沉积法制备Zn-Bi复合镀层并对其性能进行研究。结果表明,Bi可以有效地抑制电镀过程中Zn晶粒的生长,有利于镀层晶粒的细化,从而使得Zn-Bi复合镀层与纯锌镀层相比具有优异的力学性能。该方法制备的Zn-Bi复合镀层,其显微硬度是纯锌镀层的2倍,同时具有更高的耐磨性。
关键词:Zn-Bi复合镀层;离子共放电沉积;电镀;力学性能
(Edited by Xiang-qun LI)
Corresponding author: Wei GAO; Tel: +64-99238175, Fax: +64-93737463; E-mail: w.gao@auckland.ac.nz
DOI: 10.1016/S1003-6326(15)63596-8
Abstract: Zn-Bi composite was synthesized by ionic co-discharge deposition and its properties were investigated. The results show that the Zn-Bi composite with the incorporation of Bi has a finer grain size than the pure Zn coating and improves the mechanical properties. The microhardness is increased by approximately two times simply by adding a small amount of Bi electrolyte into a Zn bath solution. A lower volume loss of the Zn-Bi composite coating compared with the pure Zn coating also indicates that the Zn-Bi coating has a better wear resistance.


