
Extracting vanadium from stone-coal by oxygen pressure acid leaching and solvent extraction
DENG Zhi-gan(邓志敢), WEI Chang(魏 昶), FAN Gang(樊 刚),
LI Min-ting(李旻廷), LI Cun-xiong(李存兄), LI Xing-bin(李兴彬)
Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology,Kunming 650093, China
Received 6 July 2009; accepted 30 December 2009
____________________________________________________________________________________________
Abstract:
Vanadium extraction from stone-coal was investigated by oxygen pressure acid leaching and solvent extraction. The mineralogy of the stone-coal from Tongren City of Guizhou Province, China, was investigated by various determination methods. The effects of leaching time, leaching temperature, leaching agent concentration, leaching L/S ratio, granularity of material, additive consumption were investigated based on the mineralogy. The results show that under the conditions of leaching time of 3-4 h, temperature of 150 ℃, sulfuric acid consumption of 25%-30%, ratio of liquid to solid of 1.2:1, the granularity less than 0.074 mm, additive consumption of 3%-5%, and oxygen pressure of 1.2 MPa, and the vanadium leaching rate can be more than 92% by the method of two-step pressurized acid leaching. The powdery V2O5 product with 99.52% in V2O5 content is obtained by the flowsheet of acid recovery, removing iron by reduction process, solvent extraction, precipitating vanadium with ammonium water, and pyrolysis from the stone-coal oxygen pressure acid-leaching solution. The total recovery efficiency of vanadium is above 85%, which is more than 20% higher than that obtained in the conventional process. Furthermore, the new process does not cause air pollution since no HCl or Cl2 is released by calcination of the raw material.
Key words:
stone-coal; extracting vanadium; oxygen pressure; acid leaching; acid recovery; solvent extraction;
____________________________________________________________________________________________
1 Introduction
Vanadium is widely distributed in the nature, but it is not present alone in mineral deposit of vanadium, and it generally occurs in combination with various minerals that include carnotite, vanadium-titanium magnetite, roscoelite, vanadinite, mottramite and patronite as important sources of the metal[1-3]. There are two important vanadium sources in China; one is the vanadium and titanium magnetite ore, and the other is stone-coal ore. Stone-coal is carbonaceous shale that contains vanadium[4-8]. In China, the gross reserve of vanadium in stone-coal is 1.18?108 t in terms of V2O5, accounting for more than 87% of the domestic reserve of vanadium[9-10]. Conventionally, the extraction of vanadium from black shale is performed using the classical technology. The brief ?ow of the classical technology includes chloridizing roasting, water leaching, deposition, alkali melting and thermal decomposition. This classical technology has two main problems, that is, low recovery of vanadium (<50%) and serious environment pollution[10-11]. Serious poisonous gases, HCl and Cl2, are produced in the roasting process because sodium chloride is used as an additive, and the wastewater and solid residues contain too high concentration of heavy metals. Increasing environmental concerns and legislation concerning environmental protection, China banned the application of this technology in 2003, thus, the utilization of stone coal was limited[8-10]. Therefore, it is extremely urgent to research and develop a new technology with higher recovery of vanadium and no pollution to environment for extracting vanadium from stone-coal.
The present work focuses on the design of a new technology on vanadium extraction from stone-coal by oxygen pressure acid leaching and solvent extraction. The aim of this work is to research and develop a new process of extracting vanadium from stone-coal, characterized by higher recovery of vanadium, which was environmentally-friendly.
2 Experimental
2.1 Materials
Stone-coal was collected from Tongren City in Guizhou Province of China. The analytical grade reagents used in this study included sulfuric acid, ferrisulphas, sodium sulfite, di(2-ethylhexly)phosphoric acid (P204), tributyl phosphate (TBP), ammonia water, sodium chlorate and sodium hydroxide. All aqueous solutions were prepared using main-water. The kerosene was sulfonated with sulfuric acid (AR) to generate sulphonated-kerosene which was employed as diluent of P204 and TBP.
2.2 Methods
The mineralogy of the stone-coal from Tongren City of Guizhou Province of China was investigated by various determination methods, such as scanning electron microscopy, electron probe analysis, energy spectrum analysis, X-ray diffractometry, and thermo- gravimetric analysis. Based on the mineralogy, the effects of leaching time, leaching temperature, leaching agent concentration, leaching L/S ratio, granularity of material, additive consumption, which were the influential factors of extraction of vanadium, were investigated. The two-step countercurrent acid pressure leaching with oxygen was carried out. The powdery V2O5 product was obtained by the flowsheet of acid recovery from the stone-coal oxygen pressure acid leaching solution, removing iron by reduction process, solvent extraction, precipitating vanadium with ammonium water, and pyrolysis.
3 Results and discussion
3.1 Characterization of raw stone-coal
The X-ray diffraction pattern of stone-coal is shown in Fig.1. It can be seen from Fig.1 that the sample mainly consists of quartz, roscoelite, microcline and chromceladonite. Fig.2 shows the SEM image of stone- coal primary mineral. It can be seen from Fig.2 that the stone-coal consists of small particles with diameters below 50 ?m, and vanadium mainly exists in roscoelite, next in microcline, and iron oxide and clay mineral.
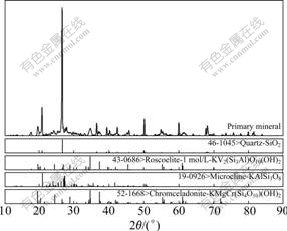
Fig.1 X-ray diffraction pattern of stone-coal

Fig.2 SEM image of stone-coal primary mineral
The composition of the mineral concentrates used in this study is listed in Table 1. It can be seen form Table 1 that the content of vanadium in terms of V2O5 is 3.26% (mass fraction, the same below if not mentioned), and reaches the industrial treatment grade.
Table 1 Main chemical components of raw materials (mass fraction, %)
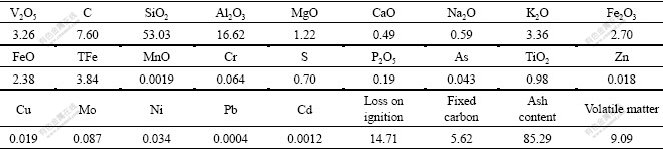
Vanadium phase and valence state in sample were analyzed by electron probe instrument. The results are listed in Table 2. It can be seen from Table 2 that the content of vanadium existing in micaceous is 1.471%, accounting for 80.55% of total vanadium; the fraction stacked to iron oxide and clay is 17.98% and the rest vanadium exists in crystal lattice of insoluble silicoaluminate mineral (1.47%).
Table 2 Distribution of vanadium in stone-coal samples
Many researches[10-14] indicated that only V3+ and V4+ were found in stone-coal generally, and V2+ and V5+
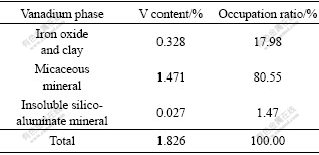
were barely found. Vanadium in the ore from major locality was mostly in the form of V3+ except for the individual region; meanwhile, V3+ replaces Al3+ in silicoaluminate as the isomorphism form, and V5+ is attached to iron oxide and clay mineral. However, as shown in Table 3, it is inconsistent with results mentioned above that the vanadium content in three forms (V3+, V4+, V5+) for sample used in this study is nearly equal and is respectively 0.627%, 0.527% and 0.672%. This indicates that the occurrence condition of vanadium in stone-coal is intricate and inconstant.
Table 3 Distribution of vanadium at different valences
3.2 Acid pressure leaching
Based on the mineralogy, to release vanadium (V3+) presented in mica mineral as the isomorphism form, the crystal lattice of mica must be broken. The reaction is as follows:
V2O3·X+2H2SO4+1/2O2→V2O2(SO4)2+2H2O+X (1)
and the speciation of VO2+ is very stable in aqueous solution. But for vanadium (V5+) presented as the absorption form, the chemical equation is as follows:
V2O5+H2SO4→(VO2)2SO4+H2O (2)
Vanadium (V4+) may present in sample as both isomorphism and absorption, the reaction[11] is
V2O2(OH)4+2H2SO4→V2O2(SO4)2+4H2O (3)
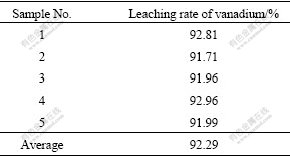
According to what has been discussed above, the investigative test was carried out. Then, orthogonal and conditional experiments were designed. The effects of leaching time, leaching temperature, leaching agent concentration, leaching L/S ratio, granularity of material, additive consumption, which were the influential factors on extraction of vanadium, were investigated[12-14]. The results showed that under the conditions of leaching time of 3-4 h, temperature of 150 ℃, sulfuric acid consumption of 25%-30%, ratio of liquid to solid of 1.2:1, the granularity less than 0.074 mm, p(O2) of 1.2 MPa and additive consumption of 3%-5%, vanadium leaching rate was over 92% by the method of two-step reverse flow oxygen pressurized acid leaching. The results of two-step countercurrent leaching are listed in Table 4.
Table 4 Results of two-step countercurrent leaching
3.3 Preprocess of leachate
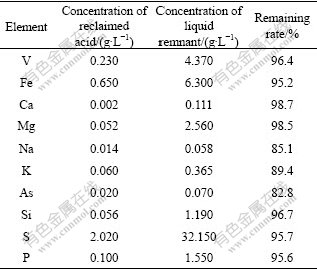
The methods of extracting vanadium from solution include oxidation-precipitation[15], direct precipitation [16] and solvent extraction[17-18]. In this work, the solvent extraction was performed to extract vanadium in the leachate produced of the two-step countercurrent leaching process, and its composition is presented in Table 5. It can be seen from Table 5 that impurity metals in solution have a high content because of the poor selectivity in acidic leaching process. This is disadvantageous for the subsequent procedure extracting vanadium pentoxide from solution [11].
Table 5 Main compositions of leaching solutions
![]()
3.3.1 Acid recovery
The high concentration of sulphuric acid in leachate would increase the addition of antalkali and ammonia, so it is very necessary to reprocess this solution before extracting vanadium. Many researches[19-21] have successfully recovered sulphuric acid from waste acid solution by diffusion dialyses method; so, the residual sulphuric acid in the solution was recovered by this method in this study. The results show that the recovery of sulphuric acid could reach above 85%, the detention rate of vanadium and impurity iron are 96% and 95%, respectively, and the sulphuric acid concentration in the reprocessed solution is about 6.5 g/L. The results of acid recovery are listed in Table 6.
Table 6 Results of acid recovery
3.3.2 Removing iron by reduction process
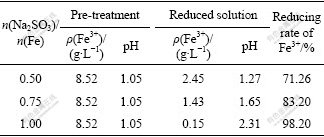
Because the extractant (P204) employed in this study can coextract vanadium and trivalence iron ion, it would lead to a low service efficiency of extractant and a product with high impurity content. Hence, it is necessary to make ferric ion reduced into ferrous ion before extracting vanadium[11, 22]. Sodium sulfite was employed as the reductant, and the addition was the same as the theoretic dosage according to the chemical equation coefficient. The reduction rate of ferric ion could reach 98.2%. The influence of sodium sulfite consumption on reduction rate of ferric iron is listed in Table 7.
Table 7 Influence of Na2SO3 consumption on reduction rate of Fe3+
3.4 Solvent extraction and anti-extraction
Some parameters of extraction and anti-extraction, such as organic phase to aqueous phase (O/A), the composition of solvent, pH and the setting time, are optimized by a series of tests[22]. The extraction experiment indicated that the 10% P204, 5% TBP and 85% sulphonated-kerosene system has a good result for extracting vanadium. The optimum extracting parameters were organic and water phase ratio (O/A) 1:2, ambient temperature, the initial pH of water phase 2.3 and contact time 10 min. Under these conditions, the extracting yield of vanadium and iron were 95.94% and 4.91%, respectively, after 6-stage counter-flow extraction with 10% P204, 5% TBP and 85% sulphonated-kerosene. The vanadium and iron concentration in the raffinate phase were 0.16 g/L and 6.00 g/L, respectively, but they were 7.56 g/L and 0.62 g/L, respectively, in the loaded organic phase.
The anti-extraction parameters were as follows: stripping agent 15% H2SO4, phase ratio (O/A) 5:1, temperature about 45 ℃, and contact time 15 min. The stripping yield of vanadium and iron could reach 99.14% and 19.35%, respectively, after 5-stage counter-flow anti- extraction. The vanadium and iron concentration in stripped organic phase were 0.065 g/L and 0.50 g/L, respectively.
By way of extraction and anti-extraction, in the stripping solution, the concentration of vanadium could be raised to 37.47 g/L or more, and the concentration of iron can be reduced to 0.6 g/L or less. The mass ratio of V to Fe can be up to 62 in aqueous phase of stripping, and the effects of separation and enrichment are well.
3.5 Precipitation and thermal decomposition
Whichever method (ion exchange resins or solvent extraction) was used in separation of vanadium from leaching solution, the vanadium was finally precipitated as ammonium vanadate by ammonia or ammonium. Because vanadium in stripping solution was V4+, the precipitation of the vanadium from the stripping solution was performed under the following conditions. 25% excess of sodium chlorate of concentration 200 g/L was added compared with the total amount of vanadium as the vanadium oxidant agent (oxidation of V(IV) to V(V)) at 60 ℃, after 1 h of oxidation, subsequently, 18% ammonia was added to keep the pH at 2 until temperature of solution was heated up to 90 ℃. Under these conditions, vanadium in stripping solution was gradually crystallized into ammonium vanadate, and the precipitation recovery of vanadium was 99.2%.
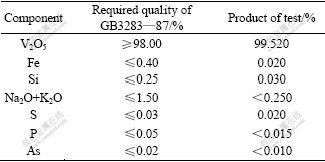
After solid liquid separation “red cake” of ammonium vanadate was thermally decomposited at 550 ℃ for 2 h to produce qualified powder vanadium pentoxide product with 99.52% in V2O5 content. The composition of powder vanadium pentoxide and product quality analyses are shown in Table 8. The overall yield of vanadium recovery could reach about 85%.
Table 8 Product quality analyses of vanadium pentoxide
4 Conclusions
1) Under the conditions of leaching time of 3-4 h, temperature of 150 ℃, sulfuric acid consumption of 25%-30%, ratio of liquid to solid of 1.2:1, the granularity less than 0.074 mm and additive consumption of 3%-5%, vanadium leaching rate could be more than 92% by the method of two-step pressurized acid leaching.
2) After pretreatment of acid recovery, removing iron by reduction process and adjusting pH of oxygen pressure acid leached solution, the extraction yield of vanadium could be up to 92% under the conditions of 6-stage counter-flow extraction with 10% P204, 5% TBP and 85% sulphonated-kerosene, and the stripping efficiency of vanadium would exceed 99% when 15% H2SO4 solution is taken as the stripping agent. By way of extraction, the concentration of vanadium could be raised to 37 g/L or more, and the concentration of iron could be reduced to 0.6 g/L or less. The mass ratio of V to Fe could be up to 60 in aqueous phase of stripping, and the effect of separation is good.
3) By this technology, vanadium could be recovered from stone-coal with recovery of 85% in the whole process, which prevents air pollution since no HCl or Cl2 is released by calcination of the raw material.
4) By this technology, the purity of the product vanadium pentoxide reaches 99.52%, which is up to the mustard quality of GB3283—87 of China.
5) The advantages of this process are: short route, simple operation, high coefficient of recovery and better economic efficiency, society benefits and environment acceptability.
References
[1] WANG Ming-yu, XIANG Xiao-yan,ZHANG Li-ping. Effect of vanadium occurrence state on the choice of extracting vanadium technology from stone coal [J]. Rare Metals, 2008, 27(2): 112-115.
[2] NAVARROA R, GUZMANA J, SAUCEDOA I, REVILLAB J, GUIBAL E. Vanadium recovery from oil fly ash by leaching, precipitation and solvent extraction processes [J]. Waste Management, 2007, 27(3): 425-438.
[3] MOSKALYK R R, ALFANTAZI A M. Processing of vanadium: A review [J]. Minerals Engineering, 2003, 16: 793-805.
[4] ZHENG Xian-ming, TIAN Xue-da, ZHANG Xiao-yun. Extraction of vanadium pentoxide from stone coal with a wet chemical separation method [J]. Natural Science Journal of Xiangtan University, 2003, 25(1): 43-45. (in Chinese)
[5] CHANG Na, GU Zhao-lin, LI Yun. Study on leaching vanadium from stone coal [J]. Chinese Journal of Inorganic Chemicals Industry, 2006, 38(7): 57-59. (in Chinese)
[6] YANG Jing-ling, JIN Xin. A new way of recovering vanadium from iron vanadium slag [J]. Journal of Beijing University of Chemical Technology, 2007, 34(3): 254-257. (in Chinese)
[7] LIANG Jian-long, LIU Hui-juan, SHI Wen-ge. A study of a new technology leaching of vanadium ores with hydrometallurgy [J]. China Mining Magazine, 2006, 15(7): 64-66. (in Chinese)
[8] HE Dong-sheng, FENG Qi-ming, ZHANG Guo-fan, OU Le-ming, LU Yi-ping. An environmentally-friendly technology of vanadium extraction from stone coal [J]. Minerals Engineering, 2007, 20(12): 1184-1186.
[9] BIN Zhi-yong. Progress of the research on extraction of vanadium pentoxide from stone coal and the market of the V2O5 [J]. Hunan Nonferrous Metals, 2006, 22(1): 16-20. (in Chinese)
[10] XIAO Wen-ding. Mineralogy of stone coal from Shanglin of Guangxi and vanadium extraction with hydrometallurgical process [J]. Nonferrous Metal, 2007, 59(3): 85-90. (in Chinese)
[11] LU Zhao-ling. Investigation and industrial practice on extraction of V2O5 from stone coal containing vanadium by acid process [J]. Hydrometallurgy of China, 2002, 21(4): 175-183. (in Chinese)
[12] LI Min-ting, WEI Chang, FAN Gang, DENG Zhi-gan. The pathbreaking experimentation study on extracting vanadium from stone-coal by acid leaching with oxygen pressure [J]. Chinese Journal of Rare Metals, 2007, 31(s): 28-31. (in Chinese)
[13] WEI Chang, FAN Gang, LI Min-ting, DENG Zhi-gan. Study on main factors effect of extracting vanadium from stone coal containing vanadium by acid leaching with oxygen pressure [J]. Chinese Journal of Rare Metals, 2007, 31(s): 98-101. (in Chinese)
[14] DENG Zhi-gan, LI Cun-xiong, WEI Chang, LI Min-ting, LIANG Yan-hui, WU Hui-ling. Research on new process of vanadium extraction from vanadium containing stone coal by acid leaching under oxygen pressure [J]. Metal Mine, 2008(7): 30-33. (in Chinese)
[15] VITOLO S, SEGGIANI M. Recovery of vanadium from heavy oil and Orimulsion fly ashes [J]. Hydrometallurgy, 2000, 57: 141-149.
[16] LOZANO L J, JUAN D. Solvent extraction of polyvanadates from sulphate solutions by Primene 81R: Its application to the recovery of vanadium from spent sulphuric acid catalysts leaching solutions [J]. Solvent Extract and Ion Exchange, 2001, 19(4): 659-676.
[17] ZHANG P W, INOUE K, YOSHIZUKA K, TSUYAMA H. Extraction and selective stripping of molybdenum (VI) and vanadium (V) from sulfuric acid solution containing aluminum (III), cobalt (II), nickel (II) and iron (III) by LIX63 in Exxsol D80 [J]. Hydrometallurgy, 1996, 41: 45-53.
[18] BAL Y, BAL K E, GOTE G, LALLAMC A. Characterization of the solid third phases that precipitate from the organic solutions of Aliquat 336 after extraction of molybdenum(Ⅵ) and vanadium(Ⅴ) [J]. Hydrometallurgy, 2004, 75: 123-134.
[19] DENG Zhi-gan, WEI Chang, LI Min-ting, LI Cun-xiong, FAN Gang, GE Huai-wen. Study on technology of extracting vanadium and removing iron from the stone-coal oxygen pressure acid-leaching solution [J]. Chinese Journal of Rare Metals, 2009, 33(2): 290-294. (in Chinese)
[20] XU Tong-wen. Recovery of acids from industrial waste liquors using anionmembrane-diffusion dialysis [J]. Technology of Water Treatment, 2004, 30(2): 63-66. (in Chinese)
[21] JEONG J K, KIM M S. Recovery of H2SO4 from waste acid solution by a diffusion dialysis method [J]. Journal of Hazardous Materials, 2005, B124: 230-232.
[22] ZHAO Yi-jiang, XING Wei-hong, XU Nan-ping. Recovery of sulfuric acid from titanium white waste acid by diffusion dialysis [J]. Journal of Chemical Engineering of Chinese Universities, 2002, 12(2): 177-200. (in Chinese)
___________________________
Foundation item: Project(2006AA06Z130) supported by the High-tech Research and Development Program of China; Project(50874053) supported by the National Natural Science Foundation of China; Project(2007GA010) supported by Science and Technology Bureau of Yunnan Province, China
Corresponding author: DENG Zhi-gan; Tel: +86-871-5188819; Fax: +86-871-5188819; E-mail: fed525700@yahoo.com.cn


