J. Cent. South Univ. Technol. (2009) 16: 0206-0211
DOI: 10.1007/s11771-009-0035-1
![]()
Self-assembly constructed by perylene bisimide derivatives bearing complementary hydrogen-bonding moieties
YANG Xin-guo(杨新国)1, YUAN Huan(袁 欢)1, ZHAO Qiu-li(赵秋丽)1,
YANG Qing(杨 青)2, CHEN Xian-hong(陈宪宏)1
(1. College of Materials Science and Engineering, Hunan University, Changsha 410082, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract:
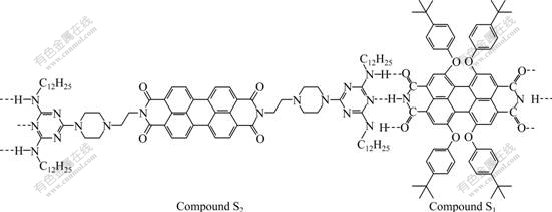
An intermediate compound 2, 4-bis(laurylamino)-6-(1-(2-aminoethyl)-piperazine)-1, 3, 5-triazine was prepared by stepwise nucleophilic substitution on triazine ring by lauryl amine and subsequently 1-(2-aminoethyl)-piperazine. Then imidization of perylene-3, 4, 9, 10-tetracarboxylic acid dianhydride with 2,4-bis(laurylamino)-6-(1-(2-aminoethyl)-piperazine)-1, 3, 5-triazine was carried out to afford a novel perylene derivative bearing two melamine blocks (S2) and 1, 6, 7, 12-tetra(4-tert-butyl phenoxy)-perylene-3, 4, 9, 10-tetracarboxylic acid bisimide (S1). The hydrogen-bonding interactions between S1 and S2 were investigated by 1H NMR spectrum, UV/Vis spectrum and fluorescence spectrum. The influences on the morphologies of S1·S2 aggregates were investigated. The results show that well-defined nanofibers with a diameter of about 100 nm can be obtained by self-assembly between S1 and S2 only in CH2Cl2 solution. Based on these results, guidelines for the molecular design and self-assembly of supramolecular polymer materials are presented.
Key words:
perylene bisimide; self-assembly; hydrogen-bonding; synthesis;
1 Introduction
The design and self-assembly of small functional molecule components into supramolecular aggregates have been one main branch of supramolecular chemistry [1-2]. An efficient control of highly mesoscopic order of molecule components by self-assembly is a subject of great importance because it is a promising avenue to developing novel functional materials and enabling the tuning of macroscopic properties of optoelectronic devices such as solar cells, light emitting diodes, and field effect transistors [3-6]. Because of the directionality and specificity of hydrogen bonding, much attention has been attracted in various supramolecular systems such as melamines with barbituric acids or imides [7-16]. The fascinating architecture and unique functionality motivate us to conceive well-defined functional nano- or mesoscopic structures from complementary molecular building blocks with the final goal of developing novel functional materials and optoelectronic devices.
Perylene derivatives are the best n-type organic semiconductors available to date. Based on their unique chemical, thermal and very high photochemical stability properties, perylene derivatives were chosen as functional building blocks for hydrogen-bonding assembly [2, 4-6, 9-18]. In this work, a novel perylene derivative containing two melamine blocks (S2) and 1, 6, 7, 12-tetra(4-tert-butylphenoxy)-perylene-3, 4, 9, 10- tetracarboxylic acid bisimide (S1) were prepared, then well-defined nanofibers were obtained by hydrogen- bonding self-assembly. The interaction of hydrogen bondings between imide in S1 and the complementary melamine moiety in S2 was investigated by 1H NMR, optical spectroscopy and electron microscopy.
2 Experimental2.1 Reagents and measurements
Perylene-3, 4, 9, 10-tetracarboxylic acid dianhydride (PTCDA) was commercial product and was purified according to the following methods: PTCDA was dissolved in 5% KOH solution after being heated, then the precipitate was collected and hydrochloric acid was added in the filtrate. The precipitate was collected, washed to be neutral with water, and dried in vacuum at 100 ℃ for 24 h.
Cyanuric chloride and methylcyclohexane (MCH) were purchased from Alfa Aesar Corporation. Other chemical reagents were purchased from the markets and purified using standard procedures.
IR spectra were measured on a Nicolet-460 spectro- meter; 1H NMR spectra were taken on an American INOVA-400 spectrometer; scanning electron micro- graphs were recorded on a JSM-6700F instrument; UV/Vis spectra were performed on a Japan UV-3010 and fluorescence spectra were measured on a Hitachi F-4500 spectrofluorometer.
2.2 Synthesis of compound S1
Perylene bisimide (S1) was synthesized according to Scheme 1 [13-15].
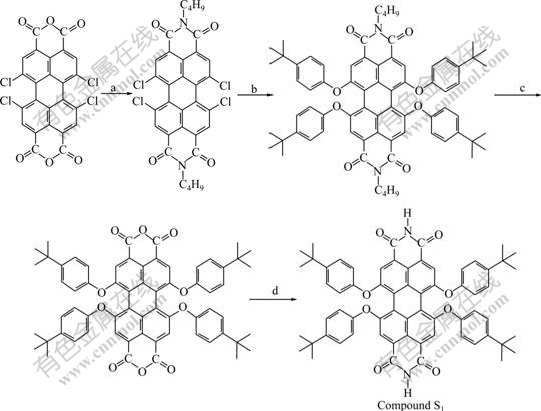
Scheme 1 Synthetic route for Compound S1: a-n-Butylamine, H2O/isopropanol, reflux, 20 h, yield of 91.0%; b-4-(Tert-butyl)phenol, K2CO3, 1-methyl-2-pyrrolidinone, 130 ℃, argon, 24 h, yield of 82.0%; c-KOH, isopropanol/H2O, reflux, argon, 48 h, yield of 80.0%; d-Ammonium acetate, propionic acid, 140 ℃, argon, 40 h, yield of 31.0%
2.3 Synthesis of compound S2
The new perylene derivative (S2) containing bismelamine chromophores was prepared according to Scheme 2 [19].
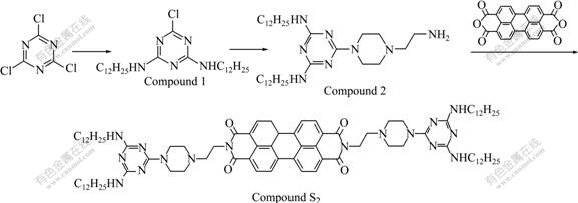
Scheme 2 Synthetic route for Compound S2
2.3.1 Synthesis of 2, 4-bis(laurylamino)-6-chloro-1, 3, 5- triazine (Compound 1)
Lauryl amine (5.0 g, 27 mmol) in CHCl3 (10 mL) was added dropwise to a solution of cyanuric chloride (2.49 g, 13.5 mmol) and N, N′-diisopropylethylamine (DIPEA) (4.86 mL, 27 mmol) in CHCl3 (50 mL). The solution was stirred at room temperature for 24 h, and 100 mL methanol was added. The resulting white precipitate was collected and redissolved in CHCl3 and then reprecipitated in methanol to give Compound 1 as a white solid (5.6 g, 85.0%). MP: 159 ℃; 1H NMR (CDCl3), chemical shift: 0.864-0.897 (t, J=6.6 Hz, 6H, CH3), 1.259-1.303 (m, 36H, CH3(CH2)9), 1.553-1.587 (m, 4H, NCH2CH2), 3.387-3.42 (t, J=6.6 Hz , 4H, NCH2), 5.407 (br s, 2H, NH); IR (KBr, cm-1): 3 252, 3 120 (υN—H), 1 640 (υC=N), 1 656 (υC=O), 1 558 (δN—H).
2.3.2 Synthesis of 2, 4-bis(laurylamino)-6-(1-(2-amino- ethyl)-piperazine)-1, 3, 5-triazine (Compound 2)
Compound 1 (4.82 g, 10 mmol) was dissolved in CHCl3 (50 mL), and 1-(2-aminoethyl)-piperazine (3.3 mL, 25 mmol) and DIPEA (0.9 mL, 5 mmol) were added. The solution was stirred at 35-40 ℃ for 24 h, and then water (100 mL) was added. The organic layer was separated and washed with water several times. The solvent was distilled off to give Compound 2 as a yellow solid that was not further purified for the following experiment.
2.3.3 Synthesis of Compound S2
Herylene derivativesMixture of Compound 2 (2.4 g, 4.2 mmol) and perylene-3, 4, 9, 10-tetracarboxylic acid dianhydride (0.494 g, 1.26 mmol) in quinoline (20 mL) were stirred at 100 ℃ for 12 h under nitrogen. After cooling to room temperature, the mixtures were poured into methanol (100 mL), and the resulting precipitate was collected and purified by column chromatography on silica eluting with chloroform-methanol (V(CHCl3)?V(CH3OH)=20?1) and reprecipitated from chloroform-methanol to obtain Compound S2 as a red solid (0.55 g, 31.0%). MP: 223 ℃; 1H NMR(CDCl3), chemical shift: 0.855-0.888 (t, J=6.6 Hz, 12H, CH3), 1.252-1.292 (m, 72H, CH3(CH2)9), 1.511-1.544 (m, 8H, NHCH2CH2), 2.633 (s, 8H, HPiper), 2.781 (s, 4H, NHCH2), 3.322 (m, 8H, NCH2), 3.758 (s, 8H, HPiper), 4.397 (s, 4H, NCH2CH2), 4.773 (br s, 4H, NH), 8.456 (s, 4H, HPery), 8.565 (s, 4H, HPery); IR(KBr, cm-1): 3 408 (υN—H), 1 691 (υC=O), 1 656 (υC=O).
3 Results and discussion
Fig.1 shows 1H NMR spectra of S1, S2 and S1·S2 (the molar ratio of S1 to S2 is 1?1) in CDCl3. The spectrum signals at 6.808-6.83, 7.227-7.249 of pure S1 are assigned to benzene ring protons, and the signal at 8.214 is assigned to perylene ring protons. The chemical shift of the imide N-H protons of pure S1 is at 8.407. In the spectrum of pure S2, the signals at 8.456, 8.565 are assigned to perylene ring protons. The equimolar mixture of S1 and S2 in CDCl3 exhibits a well-resolved spectrum with resonance of the hydrogen-bonded imide N-H protons at 9.157, benzene ring protons at 6.769-6.790, 7.219-7.241 in pure S1, and perylene protons at 8.129, 8.542-8.563, and 8.600-8.620. The electronic densities of the protons involving hydrogen bondings decrease. Consequently their 1H NMR signals shift to lower magnetic fields. These results indicate that hydrogen- bonding interactions take place between S1 and S2, which means that there exist S1·S2 complexes [14-15, 20].
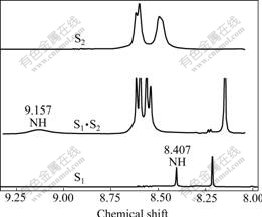
Fig.1 Partial 1H NMR spectra of S1, S2 and S1·S2 in CDCl3 (Concentration of S1 is 1 mmol/L)
The UV-Vis absorption spectra of pure S1 in CH2Cl2 at concentration of 10 μmol/L show three maxima (Fig.2(a)), which correspond to the 0-0 (578 nm), 0-1 (528 nm) and 0-2 (452 nm) transitions and are typical of monomeric perylene bisimides. The absorption spectra of pure S2 show three peaks at 524 (0-0), 488 (0-1) and 458 (0-2) nm. When the concentration of S1 is kept constant upon increasing the content of S2, the absorption intensity of S1·S2 at 578 nm, which belongs to S1, distinctly increase and no linear dependence on the content of S1 or S2 is observed. On the other hand, the relative intensities of 0-0 and 0-1 transitions for S1 and S2 in CH2Cl2 essentially unchange, which indicates that the small molecules are not aggregated in a cofacial geometry. For methylcyclohexane (Fig.2(b)), the absorption spectra of pure S1 or S2 at 50 μmol/L show the characteristics of cofacial aggregates, but the spectra of S1 and S2 mixture exhibit fine structure features of monomeric perylene derivatives to some extent. This suggests that a few three-point hydrogen bondings form between the imide group of S1 and the melamine group of S2 in methylcyclohexane, and the solubility of S1 and S2 is endowed by their complexation with each other.
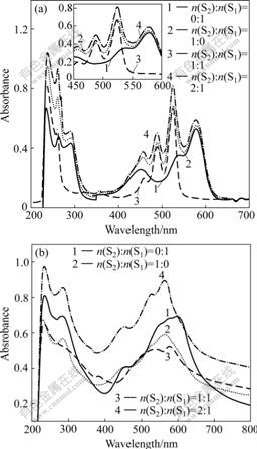
Fig.2 UV/Vis absorption spectra for stoichiometric mixture of S1 (concentration of S1 is kept at 10 μmol/L) and S2 at different molar ratios (n(S2)?n(S1)=0?1, 1?0, 1?1, 2?1) in CH2Cl2 (a) and in MCH (b)
Fig.3 shows the fluorescence spectra of pure S1, S2 and S1·S2 in CH2Cl2. The spectrum of pure S2 exhibits weaker fluorescence than that of pure S1, indicating that there is intramolecular photoinduced electron transfer between perylene bisimide chromophores and bismelamine moieties in S2. When the concentration of S1 is kept constant, the fluorescence intensities of S2 and S1 in S1·S2 complexes further decrease with the addition of S2. The formation of hydrogen bondings introduces the possibility of intermolecule electron transfer between S2 and S1 in those systems [21-23].
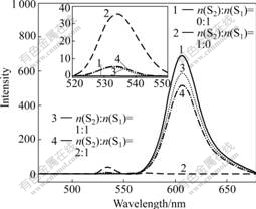
Fig.3 Fluorescence spectra (excited at 457 nm in CH2Cl2) for stoichiometric mixtures of S1 (concentration of S1 is kept constant at 10 μmol/L) and S2 at different molar ratios (n(S2)?n(S1)= 0?1, 1?0, 1?1, 2?1 )
It has been reported that S1 and melamine moieties in MCH tended to self-assembly more readily by hydrogen bondings because the high binding constants were observed in MCH [14]. As melamine moieties S2 alone or its complexation with S1 cannot disperse in MCH because of the limited solubility of S2 in MCH, the well-defined superstructures are hardly observed with scanning electron microscope (SEM) after evaporation of a 50 μmol/L MCH solution of S1?S2 (Fig.4(a)). After evaporation of a 50 μmol/L solution of S1·S2 in CH2Cl2 at room temperature, a large quantity of fibrous nano- materials (Fig.4(b)) with diameters in the range of 90-150 nm and lengths of several micrometers can be observed with SEM. No well-defined superstructures (Figs.4(c) and 4(d)) are obtained in CH2Cl2 with high concentration of S1·S2. The hydrogen-bonding interactions between the imides group of S1 and the melamines group of S2 are possibly contributed to forming 1D supramolecular structure (Scheme 3). These results are of great importance for the future studies on 1D nanomaterials in the field of photoelectric devices, and the influences of solvents and other conditions on the morphologies of S1·S2 complexes will be further investigated.
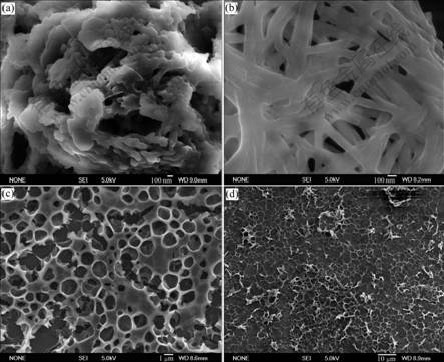
Fig.4 FE-SEM images of mesoscopic structures obtained by evaporation of 50 μmol/L solution of S1?S2 (n(S2)?n(S1)=1?1) in MCH solution (a), 50 μmol/L (b), 5 mmol/L (c) and 50 mmol/L (d) solution of S1·S2 (n(S2)?n(S1)=1?1) in CH2Cl2 solution
Scheme 3 Superstructure formation of perylene derivatives S1 with S2 by hydrogen bonding self-assembly
4 Conclusions(1) A new perylene bisimide derivative containing two melamine blocks (S2) is synthesized starting from cyanuric chloride in 3-step simple reaction with overall yield of 26.4%. The intermediates and the target compounds are characterized by IR and 1H NMR.
(2) The polarity of solvents plays an important role in self-assembly between S1 and S2. Well-defined long nanofibers with a diameter of about 100 nm are obtained by self-assembly S1 with S2 in CH2Cl2, but not in methylcyclohexane.
(3) 1H NMR spectra show that the signals at chemical shift of 8.407 of the imide N—H protons of pure S1 shift to 9.157 for S1·S2 complexes in CDCl3, indicating that hydrogen-bonding interactions take place between S1 and S2. Optical spectroscopy results suggest hydrogen-bonding interactions make a contribution to intermolecular electron transfer between S1 and S2.
References[1] BRUNSVELD L, FOLMER B J B, MEIJER E W, SIJBESMA R P. Supramolecular polymer [J]. Chem Rev, 2001, 101(12): 4071-4097.
[2] HOEBEN F J M, JONKHEIJM P, MEIJER E W, SCHENNING A P H J. About supramolecular assemblies of π-conjugated systems [J]. Chem Rev, 2005, 105(4): 1491-1546.
[3] OUALI L, KRASNIKOV V V, STALMACH U. Oligo(phenylene- vinylene)/fullerene photovoltaic cells: Influence of morphology [J]. Adv Mater, 1999, 11(14): 1515-1518.
[4] W?RTHNER F, THALACKER C, SAUTTER A, SCH?RTL W, IBACH W, HOLLRICHER O. Hierarchical self-organization of perylene bisimide-melamine assemblies to fluorescent mesoscopic superstructures [J]. Chem Eur J, 2000, 6(21): 3871-3886.
[5] ZHANG J, HOEBEN F J M, POUDEROIJEN M J, SCHENNING A P H J, MEIJER E W, de SCHRYVERF C, de FEYTERS. Hydrogen-bonded oligo(p-phenylenevinylene) functionalized with perylene bisimide: Self-assembly and energy transfer [J]. Chem Eur J, 2006, 12(35): 9046-9055.
[6] WANG Y, CHEN Y, LI R, WANG S, SU W, MA P. WASIELEWSKI M R. Amphiphilic perylenetretracarboxyl diimide dimer and its application in field effect transistor [J]. Langmuir, 2007, 23(10): 5836-5842.
[7] MORIUCHI T, TAMURA T, HIRAO T. Self-assembly of dipeptidyl ureas: A new class of hydrogen-bonded molecular duplexes [J]. J Am Chem Soc, 2002, 124(32): 9356-9357.
[8] YAGAI S, KARATSU T, KITAMURA A. Binary supramolecular gels based on bismelamine cyanurate/barbiturate noncovalent polymers [J]. Chem Mater, 2004, 16(19): 3582-3585.
[9] DEFEYTAR S, W?RTHNER F, MEIJER E W, SHENG YAO, ZHIGIAN C, WURTHNER F. Two-dimensional self-assembly into multicomponent hydrogen-bonded nanostructures [J]. Nano Lett, 2005, 5(1): 77-81.
[10] YAGAI S, MONMA Y, KAWAUCHI N, KARASTY T, KITAWURAA. Supramolecular nanoribbons and nanoropes generated from hydrogen-bonded supramolecular polymers containing perylene bisimide chromophores [J]. Org Lett, 2007, 9(6): 1137-1140.
[11] W?RTHNER F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures [J]. Chem Commun, 2004(14): 1564-1579.
[12] SINKS L E, RYBTCHINSKI B, LIMURA M, JUVES B A, GOSHE A T. Self-assembly of photofunctional cylindrical nanostructures based on perylene-3, 4, 9, 10-bis(dicarboximide) [J]. Chem Mater, 2005, 17(25): 6295-6303.
[13] W?RTHNER F, THALACKER C, SAUTTER A. Hierarchical organization of functional perylene chromophores to mesoscopic superstructures by hydrogen bonding and π-π interactions [J]. Adv Mater, 1999, 11(9): 754-758.
[14] LIU Y, ZHUANG J, LIU H, LI Y, LU F, GAN H, JIU T. Self- assembly and characterization of hydrogen-bond-induced nanostructure [J]. Chem Phys Chem, 2004, 5(8): 1210-1215.
[15] LIU Y, XIAO S, ZHU D, ZHUANG J, LU F, LI Y, XIAO S. Self-assembly and characterization of a novel hydrogen-bonded nanostructure [J]. J Phys Chem B, 2004, 108(20): 6256-6260.
[16] THALACKER C, W?RTHNER F. Chiral perylene bisimide- melamine assemblies: Hydrogen bond-directed growth of helically stacked dyes with chiroptical properties [J]. Adv Funct Mater, 2002, 12(3): 209-218.
[17] YANG Xin-guo, SUN Jing-zhi, LI Han-ying, CAO Jian, WANG Mang. Fluorescence switch based on a porphyrin-perylenediimde dyad [J]. Chinese Chemical Letters, 2005, 16(2): 257-260. (in Chinese)
[18] XIE Bo-yu, CAO Ya-feng, SUN Jing-zhi, YANG Xin-guo, WANG Mang. Change in aggregation state of a porphyrin-perylene-diimide dyad induced by trifluoroacetic acid [J]. Chinese Science Bulletin, 2007, 52(19): 2266-2270. (in Chinese)
[19] STEFFENSEN M B, SIMANEK E E. Chemoselective building blocks for dendrimers from relative reactivity data [J]. Org Lett, 2003, 5(13): 2359-2361.
[20] PRINS L J, REINHOUDT D N, TIMMEERMAN P. Non-covalent synthesis using hydrogen bonding [J]. Angew Chem Int Ed, 2001, 123(40): 2383-2426.
[21] MESSMORE B W, HULVAT J F, SONE E D, STUPP S I. Synthesis, Self-assembly, and characterization of supramolecular polymers from electroactive dendron rodcoil molecules [J]. Am Chem Soc, 2004, 126(44): 14452-14458.
[22] PRAVEEN V K, GEORGE S J, VARGHESE R, VIJAYAKUMAR C, AJAYAGHOSH A. Self-Assembled π-nanotapes as donor scaffolds for selective and thermally gated fluorescence resonance energy transfer (FRET) [J]. Am Chem Soc, 2006, 128(23): 7542-7550.
[23] GUERZO A D, OLIVE A G L, REICHWAGEN J, HOPF H, DESVERGNE J P. Energy transfer in self-assembled [n]-acene fibers involving ≥100 donors per acceptor [J]. Am Chem Soc, 2005, 127(51): 17984-17985.
Foundation item: Project(50573019) support by the National Natural Science Foundation of China
Received date: 2008-06-22; Accepted date: 2008-08-30
Corresponding author: YANG Xin-guo, Professor, PhD; Tel: +86-731-8821614; E-mail: xgyang@hnu.cn
(Edited by CHEN Wei-ping)
Abstract: An intermediate compound 2, 4-bis(laurylamino)-6-(1-(2-aminoethyl)-piperazine)-1, 3, 5-triazine was prepared by stepwise nucleophilic substitution on triazine ring by lauryl amine and subsequently 1-(2-aminoethyl)-piperazine. Then imidization of perylene-3, 4, 9, 10-tetracarboxylic acid dianhydride with 2,4-bis(laurylamino)-6-(1-(2-aminoethyl)-piperazine)-1, 3, 5-triazine was carried out to afford a novel perylene derivative bearing two melamine blocks (S2) and 1, 6, 7, 12-tetra(4-tert-butyl phenoxy)-perylene-3, 4, 9, 10-tetracarboxylic acid bisimide (S1). The hydrogen-bonding interactions between S1 and S2 were investigated by 1H NMR spectrum, UV/Vis spectrum and fluorescence spectrum. The influences on the morphologies of S1·S2 aggregates were investigated. The results show that well-defined nanofibers with a diameter of about 100 nm can be obtained by self-assembly between S1 and S2 only in CH2Cl2 solution. Based on these results, guidelines for the molecular design and self-assembly of supramolecular polymer materials are presented.
- Self-assembly constructed by perylene bisimide derivatives bearing complementary hydrogen-bonding moieties


