DOI:10.19476/j.ysxb.1004.0609.2018.06.10
AgCuZnSn钎料的热力学特性
王星星,杜全斌,彭进,崔大田,于涛源
(华北水利水电大学 机械学院,郑州450045)
摘 要:
为了揭示AgCuZnSn钎料的热力学特性,以BAg50CuZn钎料为原材料,采用熔炼合金化方法制备高锡AgCuZnSn钎料。借助差示扫描量热仪(DSC)测定不同Sn含量AgCuZnSn钎料的熔化温度,运用热分析动力学中的非等温微分法和积分法分析AgCuZnSn钎料的相变热力学特性。利用热力学熵的概念,提出AgCuZnSn钎料钎焊工艺熵和接头性能熵的数学表达式。结果表明:随着Sn含量升高,AgCuZnSn钎料的吸热峰向左偏移,且在吸热峰钎料相变温度区间变窄。非等温微分法和积分法得到的AgCuZnSn钎料的相变活化能随着Sn含量增加逐渐增大;当Sn含量相同时,两种方法得到的钎料相变活化能几乎相同。当Sn含量为7.2%(质量分数)时,AgCuZnSn钎料的相变活化能和指前因子值最大,分别为364.46 kJ/mol和7.29×1020。试验结果证实了钎焊工艺熵和接头性能熵的表达式在一定程度上可定量表征AgCuZnSn钎料的钎焊性能。
关键词:
文章编号:1004-0609(2018)-06-1159-09 中图分类号:TG454 文献标志码:A
钎料作为钎焊时的填充材料,其性能在很大程度上决定钎焊接头的质量和性能。熔化温度作为钎料性能的一项重要指标,不仅影响钎料的润湿性、熔化温度区间,而且影响钎焊过程中钎料的使用温度,进而影响钎焊接头的性能。因此,钎料熔化特性是衡量钎料钎焊性能的重要物性之一。热分析动力学作为分析合金晶型转变和相变过程的重要方法,特别是DSC技术已广泛用于多种合金相变热分析动力学特性的研 究[1-3],通过分析钎料合金的相变热力学,为研究钎料熔化特性、相变过程提供理论依据。
AgCuZnSn钎料作为一类绿色环保型钎料,在空调、眼镜、家电等制造业广泛应用,受到国内外学者的高度关注,目前,国内外对AgCuZnSn钎料的研究主要有以下几个方面:1) 通过提高Sn含量替代钎料中的Ag含量,降低钎料熔化温度,改善钎料性能[4];2) 在AgCuZnSn系钎料合金基础上,继续添加第五组元或复合添加二元及以上合金[5-8],如Ga、In、Mn、P、Ce、Ga/In、Ga-In-Ce等;3) 改进或提出钎料的制备新方法,如粉末电磁压制成形法[9]、钎焊时原位合成法[10]、镀覆扩散组合工艺[11-12]等。
已有研究认为[13],随着Sn含量升高AgCuZnSn钎料熔化温度逐渐降低,在紫铜、黄铜、纯镍表面具有很好的润湿性,钎焊接头的抗拉强度高达320 MPa。LI等[14]发现添加Sn缩小钎料熔化温度区间高达41.9 ℃;随着钎料中Sn含量升高,钎焊接头的抗拉强度升高,但Sn含量过高时接头力学性能下降。当AgCuZnSn钎料中Ag含量升高时,H62黄铜/304不锈钢钎焊接头的抗拉强度降低,接头断口呈现韧性断 裂[15]。添加10%(质量分数)的Mn代替Ag后,AgCuZnSn钎料熔化温度区间缩小,钎料中Ag含量降低10%,但Mn元素易使钎料固、液相线温度升高[16]。龙伟民等[17]通过感应加热AgCuZn/ZnCuAgSn/ AgCuZn复合焊片,在钎焊过程中原位合成AgCuZnSn钎料,该钎料中Sn含量为3.0%,同时钎缝中出现CuSn脆性相。有关高锡(Sn含量大于5.0%)AgCuZnSn钎料熔化特性热力学分析方面的研究,目前国内外还鲜见报道,该项工作对于定量分析高锡高性能银钎料的组织演变和钎焊性能具有极其重要的价值。
本文作者主要采用热分析动力学方法分析AgCuZnSn钎料的相变热力学特性,并借助熵的概念提出AgCuZnSn钎料性能的数学表达式,以期为相关领域的理论研究和工程应用提供技术支撑和科学依据。
1 实验
实验材料。以300 g的BAg50CuZn钎料(49.52% Ag,34.16% Cu,16.32% Zn)为原材料,通过成分设计、称量、熔炼、浇铸、冷却、洁净处理后,获得AgCuZnSn钎料铸锭。AgCuZnSn钎料的成分如下。1) 2.4% Sn(名义含量):48.31% Ag,33.50% Cu,15.80% Zn,2.39% Sn。2) 4.8% Sn含量:47.26% Ag,32.24% Cu,15.70% Zn,4.80% Sn。3) 5.6% Sn含量:46.98% Ag,31.83% Cu,15.61% Zn,5.58% Sn。4) 6.0% Sn含量:46.85% Ag,31.60% Cu,15.53% Zn,6.02% Sn。5) 7.2% Sn含量:46.27% Ag,31.15% Cu,15.36% Zn,7.22% Sn。
采用差示扫描量热仪(Differential scanning calorimetry,DSC)测定AgCuZnSn钎料的熔化温度,试样量为10~20 mg,所用仪器为德国NETZSCH公司的STA449F3综合热分析仪。试验在氮气保护环境下的氧化铝坩埚内完成。根据不同Sn含量银钎料的熔化温度,对钎料扫描的温度范围为30~850 ℃,升温速率20 ℃/min。利用Proteus软件对DSC扫描结果进行分析。
焊接方法。采用高频感应钎焊工艺进行对接,钎缝间隙0.05~0.06 mm,钎焊温度(750±10) ℃,时间 50 s。母材为304不锈钢(尺寸60 mm×25 mm×2.0 mm),所用钎剂为FB102,所用感应加热电源的功率35 kW,频率50 kHz。
测试分析。钎料润湿性试验根据国家标准GB/T 11364-2008《钎料润湿性试验方法》在304不锈钢表面(尺寸40 mm×40 mm×2.0 mm)测试。根据国标GB/T 11363-2008《钎焊接头强度试验方法》,利用MTS电子万能拉力试验机进行钎焊接头拉伸试验,每种Sn含量接头均测试7组,去掉最大值和最小值,取其均值。
2 结果与讨论
2.1 钎料熔化温度
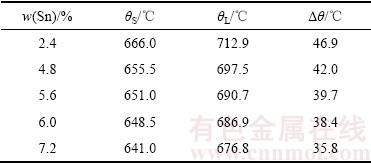
5种不同Sn含量AgCuZnSn钎料的DSC曲线,如图1所示。当升高熔化温度,钎料由固相转变为液相。设定钎料的固相线温度和液相线温度分别为DSC曲线上吸热峰的起始点温度和终止点温度。随着Sn含量升高,各钎料的吸热峰均向左偏移。图1中不同钎料对应的吸热峰特征点温度(固、液相线温度及熔化温度区间),如表1所示。Sn含量越高,钎料熔化温度区间越窄,有助于改善钎料的流动性和铺展性。
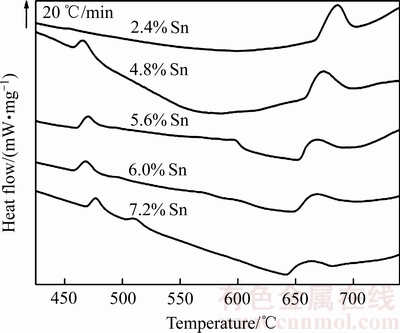
图1 5种不同Sn含量AgCuZnSn钎料的DSC曲线
Fig. 1 DSC curves of AgCuZnSn brazing alloys under five kinds of different Sn contents
表1 图1中不同吸热峰的特征点温度
Table 1 Special temperature of different endothermic peaks in Fig. 1
由图1可知,600 ℃以上5种不同Sn含量的AgCuZnSn钎料DSC曲线中仅有一个吸热峰,即在升温阶段只发生一次相变过程,故可用吸热峰揭示AgCuZnSn钎料熔化过程。升温过程中AgCuZnSn钎料由固态向液态转变的反应分数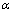 随温度的变化规律如图2所示。这里
随温度的变化规律如图2所示。这里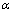 =Ht/H,DSC曲线上的数据主要表示钎料熔化过程中发生的焓变,用dHt/dt表示。
=Ht/H,DSC曲线上的数据主要表示钎料熔化过程中发生的焓变,用dHt/dt表示。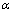 、Ht、H分别代表钎料由固态向液态转变的反应分数、钎料在t时刻的吸收热、反应完成后钎料的总吸收热。H表示DSC曲线下方的总面积,Ht表示DSC曲线下方t时刻的瞬时面积。根据图2可知,随着Sn含量升高,在吸热峰AgCuZnSn钎料的反应积分分数曲线愈加笔直,即钎料相变温度区间变窄。这说明升高Sn含量可降低AgCuZnSn钎料的固、液相线温度,缩小钎料熔化温度区间,有利于钎料由凝固态转变为熔融态。
、Ht、H分别代表钎料由固态向液态转变的反应分数、钎料在t时刻的吸收热、反应完成后钎料的总吸收热。H表示DSC曲线下方的总面积,Ht表示DSC曲线下方t时刻的瞬时面积。根据图2可知,随着Sn含量升高,在吸热峰AgCuZnSn钎料的反应积分分数曲线愈加笔直,即钎料相变温度区间变窄。这说明升高Sn含量可降低AgCuZnSn钎料的固、液相线温度,缩小钎料熔化温度区间,有利于钎料由凝固态转变为熔融态。
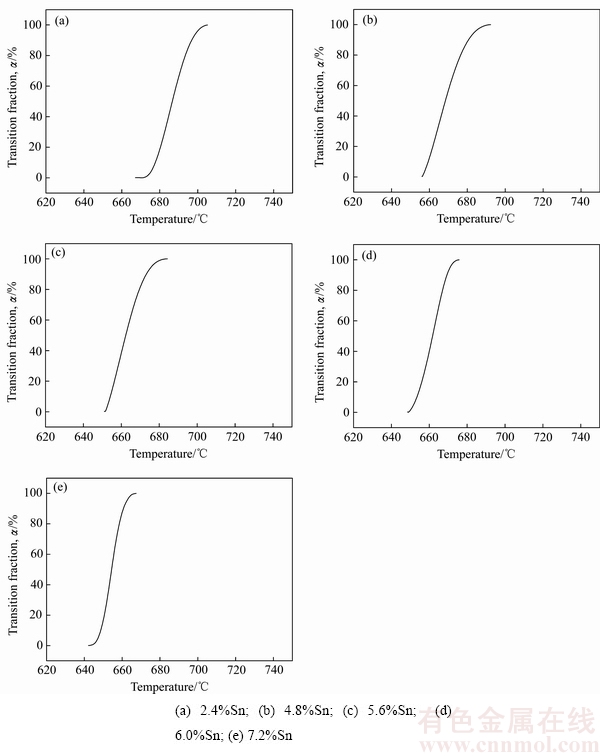
图2 Sn含量对钎料反应分数积分曲线图的影响
Fig. 2 Effect of Sn content on phase transition fraction of AgCuZnSn brazing alloys
2.2 钎料熔化特性的热力学分析
已知反应过程的微分机理方程[18]:
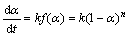 (1)
(1)
若用Arrhenius公式表示,则为
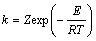 (2)
(2)
若t时刻的反应温度为
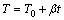 (3)
(3)
对式(3)两边同时微分,联立式(1)~(3),则
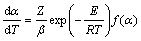 (4)
(4)
对式(4)两边取对数、微分,可得Freeman-Carroll方程
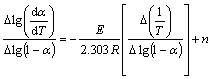 (5)
(5)
式中: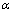 为反应分数;
为反应分数;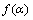 代表不同机理函数;k为反应速率常数;n为反应级数;E为活化能;Z为指前因子;R为摩尔气体常数;T为反应温度;T0为DSC测定的初始温度;β为升温速率;t为反应时间。
代表不同机理函数;k为反应速率常数;n为反应级数;E为活化能;Z为指前因子;R为摩尔气体常数;T为反应温度;T0为DSC测定的初始温度;β为升温速率;t为反应时间。
采用Origin软件将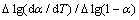 和
和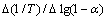 数据进行线性拟合,根据直接斜率和截距可求得E和n。
数据进行线性拟合,根据直接斜率和截距可求得E和n。
根据图1中AgCuZnSn钎料的DSC曲线与图2中反应分数积分曲线的温度数据,从固相线温度开始,步长取0.5 ℃,由不同温度对应的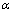 和DSC值,采用式(5)进行拟合,结果如图3所示。发现5种不同Sn含量AgCuZnSn钎料的-E/(2.303R)数值分别为-8142.28、-12159.89、-13912.01、-15093.32和-19033.64,对应的反应级数分别为0.5163、0.6810、0.4820、1.1865和1.0267,进而求得AgCuZnSn钎料的活化能E分别为155.91、232.83、266.38、289.00和364.45 kJ/mol。上述拟合结果表明,随着Sn含量逐渐升高,由非等温微分法求得的钎料相变活化能逐渐增大,同时反应级数整体呈增大趋势。主要原因可能是金属锡熔点低(232 ℃),随着Sn含量升高,钎料熔化温度区间逐渐缩小、粘度降低、流动性增强,使得钎料从凝固态转变为熔融态的速度加快。
和DSC值,采用式(5)进行拟合,结果如图3所示。发现5种不同Sn含量AgCuZnSn钎料的-E/(2.303R)数值分别为-8142.28、-12159.89、-13912.01、-15093.32和-19033.64,对应的反应级数分别为0.5163、0.6810、0.4820、1.1865和1.0267,进而求得AgCuZnSn钎料的活化能E分别为155.91、232.83、266.38、289.00和364.45 kJ/mol。上述拟合结果表明,随着Sn含量逐渐升高,由非等温微分法求得的钎料相变活化能逐渐增大,同时反应级数整体呈增大趋势。主要原因可能是金属锡熔点低(232 ℃),随着Sn含量升高,钎料熔化温度区间逐渐缩小、粘度降低、流动性增强,使得钎料从凝固态转变为熔融态的速度加快。
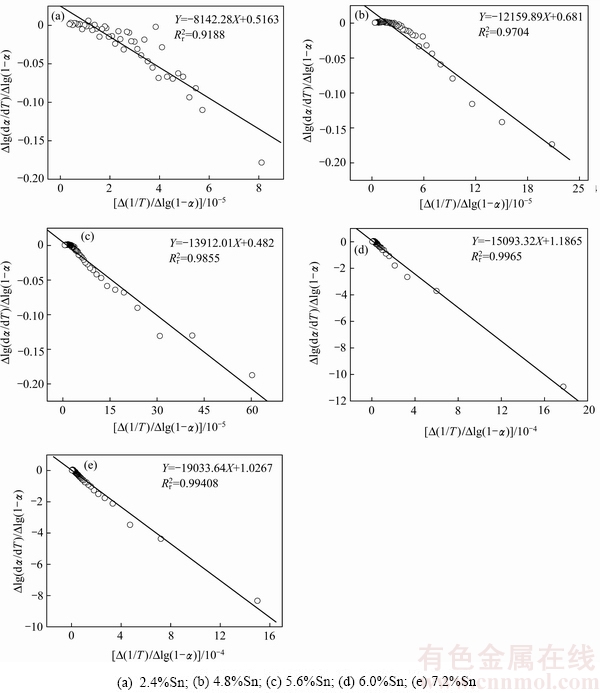
图3 Sn含量对钎料Freeman-Carroll拟合曲线图的影响
Fig. 3 Effect of Sn content on Freeman-Carroll curves of AgCuZnSn brazing alloys
热分析动力学的普适积分方程[18]为
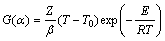 (6)
(6)
对式(6)两边同时取对数得
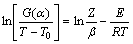 (7)
(7)
根据式(7),采用最小二乘法对不同Sn含量AgCuZnSn钎料的DSC曲线(见图1)和图2中反应分数积分曲线的温度数据进行拟合,结果如图4所示。由拟合方程可知直线的斜率-E/R,由直线截距可计算AgCuZnSn钎料的指前因子Z。经计算可知,5种不同AgCuZnSn钎料的-E/R数值分别为-18751.57、-28003.84、-32039.00、-34759.23和-43833.95,对应的钎料指前因子Z分别为5.28×109、8.44×1013、5.68×1015、9.05×1016和7.29×1020。由上述-E/R数值,求得5种AgCuZnSn钎料的活化能E分别为155.91、232.84、266.39、289.01和364.46 kJ/mol,该结果与前面非等温微分法得到的活化能E值相差甚小,几乎完全吻合。
根据图4可知,随着Sn含量逐渐升高,由非等温积分法求得的钎料相变活化能逐渐增大,同时指前因子也逐渐增大。原因在于:随着Sn含量逐渐升高,钎料熔化温度区间缩小,使得钎料从凝固态转变为熔融态的速度加快。在Sn含量为7.2%时,AgCuZnSn钎料的活化能E和指前因子Z的值最大,此时AgCuZnSn钎料的相变速率方程为k=7.29×1020·exp[-3.64×105/(RT)]。
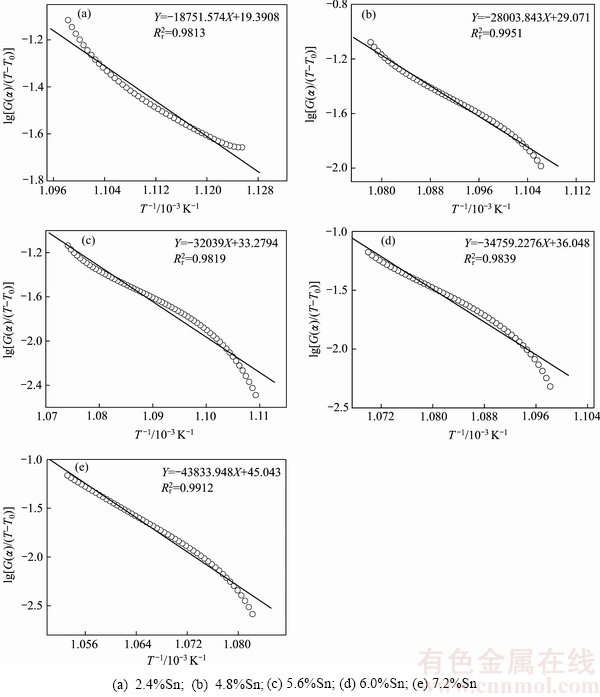
图4 Sn含量对钎料非等温积分曲线图的影响
Fig. 4 Effect of Sn content on non isothermal integral curves of AgCuZnSn brazing alloys
从金属结晶热力学角度讲,随着温度升高,固相和液相的吉布斯自由能均降低,液相的吉布斯自由能降低幅度更大[2, 19]。当固相与液相的吉布斯自由能相等时,两相同时存在,具有同样的稳定性,此时既不熔化又不结晶,处于热力学平衡状态,该温度称为理论结晶温度。当温度高于理论结晶温度时,液态金属的自由能比固态金属低,则固态金属熔化为液态。相变活化能就是金属由固态转变为液态发生相变所需的能量。而表观活化能是指采用热分析动力学方法数值求解得到的可以表征整个反应过程的活化能。
将上述非等温积分法求得的指前因子Z和活化能E代入式(2)的Arrhenius公式,可得到AgCuZnSn钎料相变过程中相变速率常数k在相变温度范围内的变化规律。再将式(2)两边取对数,可得到AgCuZnSn钎料相变速率常数k与温度T之间的关系lnk=lnZ-E/(RT),对AgCuZnSn钎料的相变速率与温度关系进行拟合,结果如图5所示。
根据图5可知,温度越高,相变速率常数越大,钎料由固相转变为液相的速度越快;反之,转变速度越慢。随着Sn 含量升高,图中拟合直线的斜率减小,说明AgCuZnSn钎料的相变速率常数k减小,表明钎料由固相转变为液相的速度减缓。
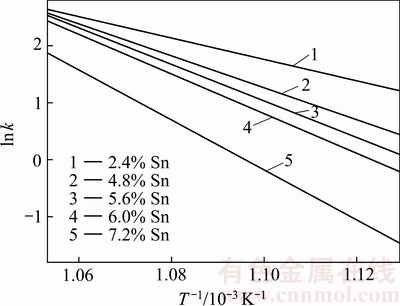
图5 Sn含量对AgCuZnSn钎料相变速率与温度拟合曲线图的影响
Fig. 5 Effect of Sn content on phase transformation rate of AgCuZnSn brazing alloys and temperature
2.3 钎料钎焊性能的定量表征
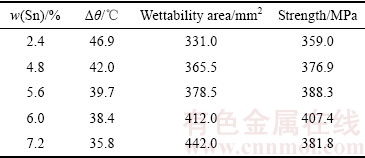
以BAg50CuZn钎料为原料,熔炼合金化方法制备的AgCuZnSn钎料钎焊工艺性和接头力学性能的试验数据,如表2所列。钎焊接头抗拉强度不低于350 MPa,钎料润湿面积不小于330 mm2,熔化温度区间小于50 ℃。这表明随着Sn含量升高,钎料在304不锈钢表面的润湿面积均增大,钎料熔化温度区间缩小,钎焊接头的抗拉强度先升高后降低。对比表2中的试验数据发现,钎料熔化温度区间、润湿面积、接头力学性能与钎料中Sn含量的变化规律不一致。因此,建立统一的数学模型,预测不同Sn含量AgCuZnSn的钎焊性能是分析的关键。
表2 钎料钎焊工艺性及其接头力学性能的试验数据
Table 2 Brazability and brazed joints mechanical properties of AgCuZnSn brazing alloys
热力学中将可逆过程中物质系统吸收的热量与绝对温度的比值d(Q/T),称为熵的增量(dS)。熵是物质热力学状态的函数,与物质热力学状态变化的路径无关[20-21]。熵越小,可转变程度越高,不可转变程度越低;反之,不可转变程度越高。同时,熵具有方向性。所以,通过研究熵变,可对钎料性能变化趋势作出较为精确的预测。
利用熵的概念,将AgCuZnSn钎料的钎焊工艺性和钎焊接头的力学性能统一用熵值表达,熵值越大表示对应钎料的钎焊工艺性和钎焊接头的力学性能越差;反之,钎焊工艺性和接头力学性能愈好。将AgCuZnSn钎料的钎焊工艺性和接头力学性能与熵的关系分别用两个数学公式表示,见下面式(8)和式(9)。其中Δθ代表钎料熔化温度区间,wSn代表钎料中Sn含量。
SG(钎焊工艺熵)表征钎料钎焊工艺性的熵,对应其铺展系数(黏度)和熔化温度区间。SG的倒数与润湿面积(或黏度)正相关,与熔化温度区间负相关。
SX(接头性能熵)表征钎焊接头力学性能的熵,对应其抗拉强度(抗剪强度)。SX的倒数与抗拉强度(抗剪强度)正相关。
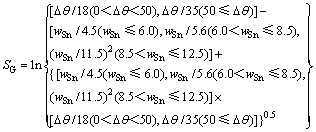 (8)
(8)
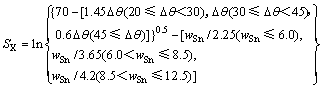 (9)
(9)
利用表2中试验数据根据式(8)进行计算,获得AgCuZnSn钎料钎焊工艺熵值的倒数、润湿面积与钎料中Sn含量的变化规律,如图6所示。将图6中两条曲线对比可知,随着Sn含量升高,AgCuZnSn钎料对应的钎焊工艺熵值的倒数越大,即工艺熵值越小,对应的钎料润湿面积越大、熔化温度区间越窄,钎料的润湿性愈好。原因在于:金属Sn的熔点为232 ℃,远低于银基钎料的熔化温度。对于AgCuZnSn钎料,通过熔炼合金化方法添加Sn后,钎料组织中出现Ag3Sn、Cu3Sn低熔点化合物相,根据Ag-Sn和Cu-Sn二元相图,Cu3Sn和Ag3Sn相的熔点分别为415和480 ℃,远低于AgCuZnSn钎料的熔化温度,且弥散分布。正是由于这两种相的存在使得AgCuZnSn钎料固、液相线温度降低,相变速率加快。由表2可知,随着钎料中Sn含量升高,AgCuZnSn钎料熔化温度区间逐渐变窄,使得公式(8)中工艺熵值SG较小,故其倒数较大,钎料润湿性越好。进一步分析图6可知,随着Sn含量升高,钎料工艺熵值的变化趋势与钎料润湿性的变化规律基本吻合,表明提出的钎焊工艺熵的数学表达式在一定程度上可定量表征AgCuZnSn钎料的钎焊工艺性。
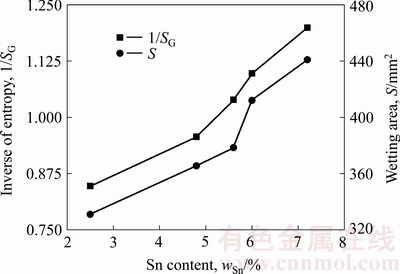
图6 Sn含量对钎焊工艺熵和钎料润湿面积的影响
Fig. 6 Effect of Sn content on process entropy and wetting area of brazing alloys
同样,利用表2中试验数据由式(9)进行计算,得到的接头性能熵值的倒数及钎焊接头抗拉强度与Sn含量的变化规律,如图7所示。分析两条曲线的整体变化趋势可知,随着Sn含量升高,AgCuZnSn钎料对应接头性能熵值的倒数越大,即接头性能熵值越小,对应304不锈钢钎焊接头的力学性能愈好。在Sn含量为6.0%时,304不锈钢接头性能熵值的倒数最大,即性能熵值最小,钎焊接头抗拉强度最高。进一步分析图7中可知,随着Sn含量升高,接头性能熵值的变化趋势与钎焊接头抗拉强度的变化趋势一致,这表明提出的钎焊接头性能熵的数学表达式(9)也在一定程度上可以定量表征AgCuZnSn钎料钎焊接头的力学性能。
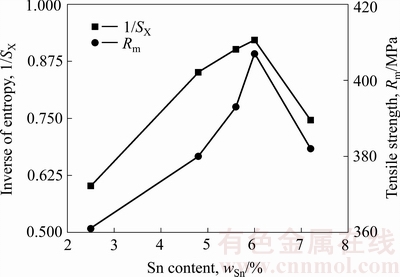
图7 Sn含量对接头性能熵和抗拉强度的影响
Fig. 7 Effect of Sn content on performance entropy and tensile strength of brazed joints
3 结论
1) 随着Sn含量升高,AgCuZnSn钎料的吸热峰向左偏移,钎料熔化温度区间缩小;在吸热峰AgCuZnSn钎料的相变温度区间变窄,但高锡AgCuZnSn钎料的相变速率常数随着Sn含量升高逐渐减小。
2) 采用非等温微分法和积分法对AgCuZnSn钎料的相变热力学特性进行了分析。随着Sn含量逐渐升高,两种方法得到的钎料相变活化能均逐渐增大;同等Sn含量条件下,两种方法得到的钎料相变活化能几乎完全相同。
3) 在Sn含量为7.2%时,AgCuZnSn钎料的活化能和指前因子值最大,分别为364.46 kJ/mol和7.29×1020。此时钎料相变速率方程的表达式为k=7.29×1020exp[-3.64×105/(RT)]。
4) 提出了高锡AgCuZnSn钎料钎焊工艺熵SG和接头性能熵SX的数学表达式。
5) 随着Sn含量的升高,钎焊工艺熵值和接头性能熵值均逐渐减小,试验结果证实钎焊工艺熵和接头性能熵的数学表达式在一定程度上可定量表征AgCuZnSn钎料的钎焊工艺性和钎焊接头的力学性能。
REFERENCES
[1] LEE B J, WANG N M, LEE H M. Prediction of interface reaction productions between Cu and various solder alloys by thermodynamic calculation[J]. Acta Materialia, 1997, 45(5): 1867-1874.
[2] APEL M, LASCHET G,  B, BERGER R. Phase field modeling of microstructure formation, DSC curves, and thermal expansion for Ag-Cu brazing fillers under reactive air brazing conditions[J]. Advanced Engineering Materials, 2014, 16(12): 1468-1474.
B, BERGER R. Phase field modeling of microstructure formation, DSC curves, and thermal expansion for Ag-Cu brazing fillers under reactive air brazing conditions[J]. Advanced Engineering Materials, 2014, 16(12): 1468-1474.
[3] BAO Li, LONG Wei-min, HE Peng, WU Ming-fang, GU Xiao-long, MA Jia. Effect of trace calcium on melting behavior of Ag-Cu-Zn brazing alloy by thermal analysis kinetics[J]. China Welding (English Edition), 2015, 24(4): 15-20.
[4] 王 禾, 薛松柏, 刘 霜. 银元素对含银钎料性能的影响[J]. 中国有色金属学报, 2016, 26(11): 2340-2352.
WANG He, XUE Song-bai, LIU Xiang. Effect of Ag on properties of Ag-contained filler metals[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(11): 2340-2352.
[5] WINIOWSKI A,  M. Impact of tin and nickel on the brazing properties of silver filler metals and on the strength of brazed joints made of stainless steels[J]. Archives of Metallurgy and Materials, 2013, 58(4): 1007-1011.
M. Impact of tin and nickel on the brazing properties of silver filler metals and on the strength of brazed joints made of stainless steels[J]. Archives of Metallurgy and Materials, 2013, 58(4): 1007-1011.
[6] WATANABE T, YANAGISAWA A, SASAKI T. Development of Ag based brazing filler metal with low melting point[J]. Science and Technology of Welding and Joining, 2011, 16(6): 502-508.
[7] LAI Zhong-min, XUE Song-bai, HAN Xian-peng, GU Li-yong, GU Wen-hua. Study on microstructure and property of brazed joint of AgCuZn-X(Ga, Sn, In, Ni) brazing alloy[J]. Rare Metal Materials and Engineering, 2010, 39(3): 397-400.
[8] MA Chao-li, XUE Song-bai, WANG Bo. Study on novel Ag-Cu-Zn-Sn brazing filler metal bearing Ga[J]. Journal of Alloys and Compounds, 2016, 688: 854-862.
[9] 高 歌, 胡建华, 程 呈, 吴 轩, 张 达. 电磁压制多元金属混合粉末的压型方程[J]. 中国有色金属学报, 2015, 25(7): 1937-1942.
GAO Ge, HU Jian-hua, CHENG Cheng, WU Xuan, ZHANG Da. Forming equation about multivariate mixed metal powder by electromagnetic compaction[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(7): 1937-1942.
[10] LONG W M, ZHANG G X, ZHANG Q K. In situ synthesis of high strength Ag brazing filler metals during induction brazing process[J]. Scripta Materialia, 2016, 110: 41-43.
[11] 王星星, 龙伟民, 马 佳, 吕登峰. 锡镀层对BAg50CuZn钎料性能的影响[J]. 焊接学报, 2014, 35(9): 61-64.
WANG Xing-xing, LONG Wei-min, MA Jia, LV Deng-feng. Effect of electroplated tin coating on properties of BAg50CuZn brazing filler metal[J]. Transactions of the China Welding Institution, 2014, 35(9): 61-64.
[12] 王星星, 彭 进, 崔大田, 杜全斌, 王建升. 银基钎料锡电镀层的界面特征分析[J]. 中国有色金属学报, 2017, 27(10): 2053-2061.
WANG Xing-xing, PENG Jin, CUI Da-tian, DU Quan-bin, WANG Jian-sheng. Analysis of interface characteristic for tin electroplating coating on silver brazing filler metals[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(10): 2053-2061.
[13] WIERZBICKI L J, MALEC W, STOBRAWA J, CWOLEK B, JUSZCZYK B. Studies into new, environmentally friendly Ag-Cu-Zn-Sn brazing alloys of low silver content[J]. Archives of Metallurgy and Materials, 2011, 56(1): 147-158.
[14] LI M G, SUN D Q, QIU X M, YIN S Q. Effect of tin on melting temperature and microstructure of Ag-Cu-Zn-Sn filler metals[J]. Materials Science and Technology, 2005, 21(11): 1318-1322.
[15] CAO J, ZHANG L X, WANG H Q, WU L Z, FENG J C. Effect of silver content on microstructure and properties of brass/steel induction brazing joint using Ag-Cu-Zn-Sn filler metal[J]. Journal of Materials Science & Technology, 2011, 27(4): 377-381.
[16] DANIEL S, GUNTHER W, SEBASTIAN S. Development of Ag-Cu-Zn-Sn brazing filler metals with a 10weigh-% reduction of silver and liquids temperature[J]. China Welding (English Edition), 2014, 23(4): 25-31.
[17] 龙伟民, 张冠星, 张青科, 何 鹏, 薛 鹏. 钎焊过程原位合成高强度银钎料[J]. 焊接学报, 2015, 36(11): 1-4.
LONG Wei-min, ZHANG Guan-xing, ZHANG Qing-ke, HE Peng, XUE Peng. In-situ synthesis of high strength Ag brazing filler metals during brazing process[J]. Transactions of the China Welding Institution, 2015, 36(11): 1-4.
[18] 胡荣祖, 史启祯. 热分析动力学[M]. 北京: 科学出版社, 2001: 64-66.
HU Rong-zu, SHI Qi-zhen. Kinetic of thermal analysis[M]. Beijing: Science Press, 2001: 64-66.
[19] GANCARZ T, PSTRUS J. Formation and growth of intermetallic phases at the interface in the Cu/Sn-Zn-Ag-Cu/Cu joints[J]. Journal of Alloys and Compounds, 2015, 647: 844-856.
[20] EL MANIANI M, SABBAR A. Partial and integral enthalpies of mixing in the liquid Ag-In-Sn-Zn quaternary alloys[J]. Thermochimica Acta, 2014, 592: 1-9.
[21] BENISEK A, DACHS E. A relationship to estimate the excess entropy of mixing: Application in silicate solid solutions and binary alloys[J]. Journal of Alloys and Compounds, 2012, 527: 127-131.
Thermodynamics characteristics of AgCuZnSn brazing filler metals
WANG Xing-xing, DU Quan-bin, PENG Jin, CUI Da-tian, YU Tao-yuan
(School of Mechanical Engineering, North China University of Water Resources and Electric Power, Zhengzhou 450045, China)
Abstract: By revealing the thermodynamic properties of AgCuZnSn brazing alloys, brazing alloys of high Sn content were prepared using melting alloyed method based on BAg50CuZn substrate. The melting temperature of brazing alloys was observed by differential scanning calorimeter (DSC), and its phase transformation thermodynamic characteristic was analyzed with the thermal analysis kinetics of non-isothermal differential and integral methods. The mathematical expression of process entropy and performance entropy of brazing alloys were proposed. The results show that the DSC endothermic peak of AgCuZnSn brazing alloys shifts to the left, and its phase transformation temperature interval would be narrower with the increase of Sn content. The phase transition activation energy of AgCuZnSn brazing alloys gradually increases using non-isothermal methods. Under the same Sn content, the phase transition activation energy of brazing alloys with non-isothermal differential method is exactly the same as that of integral method. When Sn content is 7.2% (mass fraction), the transition activation energy and pre-exponential factor of brazing alloys reach the maximum, which are 364.46 kJ/mol and 7.29×1020, respectively. The results indicate that the expression of process entropy and performance entropy could quantitative analyze the brazability of AgCuZnSn brazing alloys.
Key words: silver brazing alloy; melting characteristic; thermodynamic entropy; activation energy
Foundation item: Project(51705151) supported by the National Natural Science Foundation of China; Project (162300410191) supported by the Natural Science Foundation of Henan Province, China; Project(17A430021) supported by the Universities Key Scientific Research Projects of Henan Province, China; Project(201810078027) supported by the Innovation Training Program for College Students in Henan Province, China
Received date: 2017-05-02; Accepted date: 2017-11-03
Corresponding author: WANG Xing-xing; Tel: +86-371-69127295; E-mail: paperwxx@126.com
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51705151);河南省自然科学基金资助项目(162300410191);河南省高等学校重点科研项目(17A430021);河南省高等学校大学生创新训练计划项目(201810078027)
收稿日期:2017-05-02;修订日期:2017-11-03
通信作者:王星星,讲师,博士;电话:0371-69127295;E-mail: paperwxx@126.com
摘 要:为了揭示AgCuZnSn钎料的热力学特性,以BAg50CuZn钎料为原材料,采用熔炼合金化方法制备高锡AgCuZnSn钎料。借助差示扫描量热仪(DSC)测定不同Sn含量AgCuZnSn钎料的熔化温度,运用热分析动力学中的非等温微分法和积分法分析AgCuZnSn钎料的相变热力学特性。利用热力学熵的概念,提出AgCuZnSn钎料钎焊工艺熵和接头性能熵的数学表达式。结果表明:随着Sn含量升高,AgCuZnSn钎料的吸热峰向左偏移,且在吸热峰钎料相变温度区间变窄。非等温微分法和积分法得到的AgCuZnSn钎料的相变活化能随着Sn含量增加逐渐增大;当Sn含量相同时,两种方法得到的钎料相变活化能几乎相同。当Sn含量为7.2%(质量分数)时,AgCuZnSn钎料的相变活化能和指前因子值最大,分别为364.46 kJ/mol和7.29×1020。试验结果证实了钎焊工艺熵和接头性能熵的表达式在一定程度上可定量表征AgCuZnSn钎料的钎焊性能。
[4] 王 禾, 薛松柏, 刘 霜. 银元素对含银钎料性能的影响[J]. 中国有色金属学报, 2016, 26(11): 2340-2352.
[9] 高 歌, 胡建华, 程 呈, 吴 轩, 张 达. 电磁压制多元金属混合粉末的压型方程[J]. 中国有色金属学报, 2015, 25(7): 1937-1942.
[11] 王星星, 龙伟民, 马 佳, 吕登峰. 锡镀层对BAg50CuZn钎料性能的影响[J]. 焊接学报, 2014, 35(9): 61-64.
[12] 王星星, 彭 进, 崔大田, 杜全斌, 王建升. 银基钎料锡电镀层的界面特征分析[J]. 中国有色金属学报, 2017, 27(10): 2053-2061.
[17] 龙伟民, 张冠星, 张青科, 何 鹏, 薛 鹏. 钎焊过程原位合成高强度银钎料[J]. 焊接学报, 2015, 36(11): 1-4.
[18] 胡荣祖, 史启祯. 热分析动力学[M]. 北京: 科学出版社, 2001: 64-66.
HU Rong-zu, SHI Qi-zhen. Kinetic of thermal analysis[M]. Beijing: Science Press, 2001: 64-66.


