
Electronic structure and physical properties of hcp Ti3Al type alloys
PENG Hong-jian(彭红建)1, 2, XIE You-qing(谢佑卿)2
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. School of Materials Science and Engineering, Central South University, Changsha 410083, China
Received 20 October 2006; accepted 9 April 2007
Abstract:
According to the basic information of sequences of Ti and Al characteristic atoms in hcp Ti-Al system, the compositional variations of the electronic structure, atomic potential energies, atomic volumes, lattice constants and cohesive energies of the ordered hcp Ti3Al type alloys were calculated by the framework of systematic science of alloys(SSA). The electronic structure of the hcp Ti3Al compound consisted of ![]() and
and ![]() atoms is 0.75[Ar] (3dn)0.573(3dc)2.1685(4sc)0.972(4sf)0.3093+0.25[Ne](3sc)1.32? (3pc)1.19(3sf)0.49. The factors of controlling lattice stability are electronic structure, atomic energies and atomic concentration. The
atoms is 0.75[Ar] (3dn)0.573(3dc)2.1685(4sc)0.972(4sf)0.3093+0.25[Ne](3sc)1.32? (3pc)1.19(3sf)0.49. The factors of controlling lattice stability are electronic structure, atomic energies and atomic concentration. The ![]() atoms play a determinative role in forming D019 structure with a=0.287 2 nm, c=0.456 4 nm, atomic cohesive energy ε=4.810 8 eV/atom and heat of formation ΔH=-0.332 8 eV/atom. These calculated values are in good agreement with experimental values (a=0.287 5 nm, c=0.46 0 nm, ?H=-0.27, -0.29 eV/atom). The calculated cohesive energy of the hcp Ti3Al compound is slightly bigger than that of the fcc Ti3Al.This is a good sign that makes it feasible to stabilized L12 structure of the hcp Ti3Al compound by ternary element. The new element should have more dc-electrons than Ti-metal and occupy at the Ti-lattice points.
atoms play a determinative role in forming D019 structure with a=0.287 2 nm, c=0.456 4 nm, atomic cohesive energy ε=4.810 8 eV/atom and heat of formation ΔH=-0.332 8 eV/atom. These calculated values are in good agreement with experimental values (a=0.287 5 nm, c=0.46 0 nm, ?H=-0.27, -0.29 eV/atom). The calculated cohesive energy of the hcp Ti3Al compound is slightly bigger than that of the fcc Ti3Al.This is a good sign that makes it feasible to stabilized L12 structure of the hcp Ti3Al compound by ternary element. The new element should have more dc-electrons than Ti-metal and occupy at the Ti-lattice points.
Key words:
Ti3Al alloy; electronic structure; physical property;
1 Introduction
The intermetallic compound hcp Ti3Al with D019 structure has been the subject of considerable attention by experimentalists[1-3]. It is considered as a desirable candidate for application in aircraft turbine engines because its static strength and stiffness do not degrade rapidly as temperature increase. The calculations of the Ti-Al phase diagram using a variety of models have been performed[4-8]. But these Gibbs energy functions do not be associated with electronic structure, atomic energies, atomic volumes and lattice constants. The knowledge of the Ti-Al system obtained from Gibbs energy functions is not complete.
According to the framework of systematic science of alloys(SSA)[9], the Ti-Al system has fcc, hcp and bcc lattice systems, besides a liquid system. It has been found that the compositional variations of the electronic structure, atomic potential energies, atomic volumes, lattice constants, cohesive energies and Gibbs energies of these four phases can be calculated from the basic information of the same sequences of the characteristic atoms in the fcc Ti-Al lattice system[10-13].
In this study, the basic information of the sequences of Ti and Al characteristic atoms in the hcp Ti-Al lattice system was determined. The properties of hcp Ti3Al alloys as a function of composition were obtained and the controlling factors for crystalline stability of hcp Ti3Al compound were analyzed.
2 Basic information of Ti and Al characteri- stic atoms
According to the basic clusters overlapping(BCO) model in SSA framework, there are two basic cluster sequences that include 13 kinds of Ti and Al basic clusters in hcp Ti-Al system. The disordered and ordered alloys with hcp Ti3Al type can be formed by arrangements of these 26 kinds of characteristic atoms, or by mixing the following 26 kinds of characteristic crystals. According to information about experimental lattice constants of some hcp TixAl(1-x) alloys and on the basis of experimental value and theoretical analysis, we have determined the basic information about the electronic structures, volumes, lattice constants, potentialenergies and cohesive energies of the characteristic atoms and corresponding characteristic crystals in the hcp Ti-Al system (Tables 1 and 2).
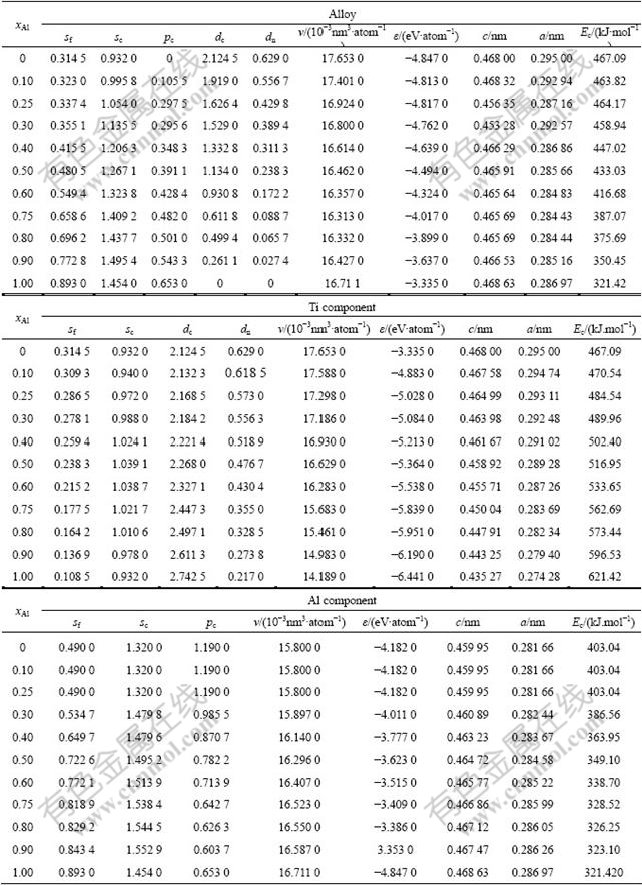
Table 1 Structural parameters and properties of Ti-characteristic atoms and Ti-characteristic crystals in hcp Ti-Al system
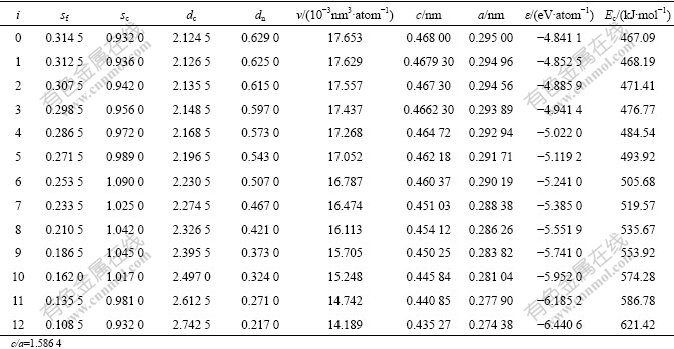
Table 2 Structural parameters and properties of Al-characteristic atoms and Al-characteristic crystals in hcp Ti-Al system
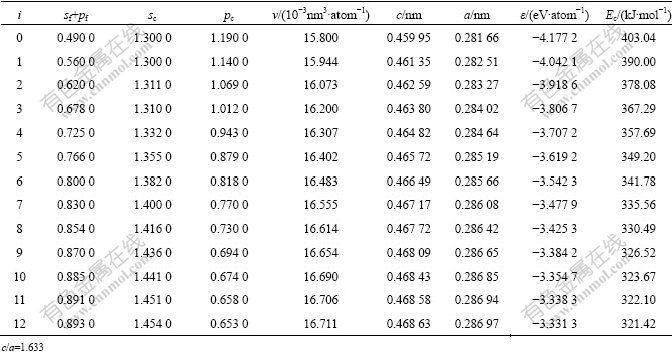
In Tables 1 and 2, sc, pc and dc are respectively the covalent electrons in s, p and d orbitals, sf and pf are the near-free electrons in s and p orbitals, and dn is the nonbonded electrons in d orbital.
As shown in Fig.1, for hcp Ti-Al system, the relationships of the ![]() ,
, ![]() ,
, ![]() and
and ![]() with the number i can be described by following equation, where Q can respectively denote ε and v.
with the number i can be described by following equation, where Q can respectively denote ε and v.
![]() (1)
(1)
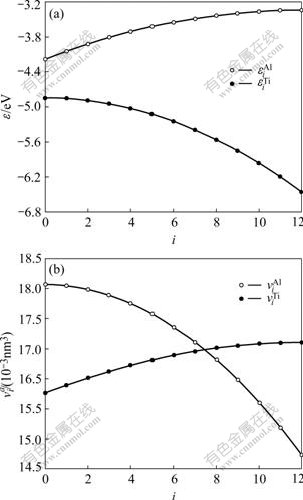
Fig.1 Relationships of![]() and
and ![]() (a),
(a), ![]() and
and ![]() (b) of
(b) of ![]() and
and ![]() atoms with number i of nearest neighbor Al atoms in hcp Ti-Al system
atoms with number i of nearest neighbor Al atoms in hcp Ti-Al system
3 Basic information of ordered hcp Ti3Al type alloys
Since the complex interactions between atoms in the alloys are reflected by the electronic structure, potential energies, atomic volumes, lattice constants, cohesive energies and Gibbs energies of the characteristic crystals, the basic information of the alloys can be obtained by the additive law of characteristic crystals (Eqn.(2)):
 (2)
(2)
where ![]() and
and ![]() are concentrations of the Ti and Al characteristic atoms in the alloys.
are concentrations of the Ti and Al characteristic atoms in the alloys.
For the ordered hcp Ti3Al type alloys with maximal ordering degree, the concentrations ![]() and
and ![]() as a function of composition are shown in Fig.2. The basic information about the compositional variations of electronic structure, atomic potential energies, atomic volumes, lattice constants and cohesive energies of the hcpTi3Al alloys and their components Ti and Al are listed in Table 3. It can be known that the hcp Ti3Al compound is formed by
as a function of composition are shown in Fig.2. The basic information about the compositional variations of electronic structure, atomic potential energies, atomic volumes, lattice constants and cohesive energies of the hcpTi3Al alloys and their components Ti and Al are listed in Table 3. It can be known that the hcp Ti3Al compound is formed by ![]() and
and ![]() atoms.
atoms.
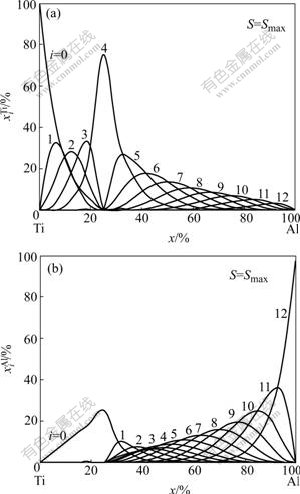
Fig.2 Concentrations![]() and
and ![]() of ordered TixAl(1-x) alloys with hcp Ti3Al-type and maximal ordering degree as functions of composition xAl
of ordered TixAl(1-x) alloys with hcp Ti3Al-type and maximal ordering degree as functions of composition xAl
Table 3 Electronic structure and properties of ordered TixAl1-x alloys with hcp Ti3Al-type having maximal ordering degree and their components of Ti and Al
4 Crystalline structures of Ti3Al type alloys
4.1 Relationship of lattice stability with electronic structure for Ti3Al compound
The factors of controlling lattice stability are electronic structure, atomic energy and atomic concentration. The perfectly ordered Ti0.75Al0.25 alloy may be hcp Ti3Al compound with D019 structure, or fcc Ti3Al compound with L12 structure.
In order to understand the effect of electronic structure factor on lattice stability, the average electronic structures of hcp Ti3Al and fcc Ti3Al[12] compounds are shown as follows:
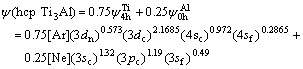 (3)
(3)
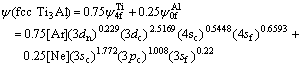 (4)
(4)
It should be pointed out that the ![]() and
and ![]() atoms belong in the sequences of Ti- and Al-characteristic atoms in the hcp lattice system, and the
atoms belong in the sequences of Ti- and Al-characteristic atoms in the hcp lattice system, and the ![]() and
and ![]() atoms belong in the sequences of Ti- and Al-characteristic atoms in the fcc lattice system.
atoms belong in the sequences of Ti- and Al-characteristic atoms in the fcc lattice system.
The ![]() atoms have a tendency to form hcp and bcc structures, because their dc electrons would rather be in the eg state with lower potential energy than in the t2g state with higher potential energy. Only when the Al atoms or other elements have stronger tendency to form fcc structure than the tendency to form hcp and bcc structure of the Ti atoms, can the dc electrons be in the t2g state due to strong effect of Al atoms, and can the Ti3Al compounds have fcc lattice structure.
atoms have a tendency to form hcp and bcc structures, because their dc electrons would rather be in the eg state with lower potential energy than in the t2g state with higher potential energy. Only when the Al atoms or other elements have stronger tendency to form fcc structure than the tendency to form hcp and bcc structure of the Ti atoms, can the dc electrons be in the t2g state due to strong effect of Al atoms, and can the Ti3Al compounds have fcc lattice structure.
The ![]() atoms have a tendency to form fcc structure. The
atoms have a tendency to form fcc structure. The ![]() atoms in the fcc Ti3Al compound have less concentration (
atoms in the fcc Ti3Al compound have less concentration (![]() =25%, mole fraction), less sc spherical covalent electrons and more pc directional electrons (1.008 eV/atom) than other atoms. Thereby, the
=25%, mole fraction), less sc spherical covalent electrons and more pc directional electrons (1.008 eV/atom) than other atoms. Thereby, the ![]() atoms have weaker role in forming fcc lattice than other atoms in the fcc TiAl3 compounds.
atoms have weaker role in forming fcc lattice than other atoms in the fcc TiAl3 compounds.
The ![]() atoms have the most concentration (
atoms have the most concentration (![]() =75%, mole fraction) and the strongest tendency to form hcp and bcc structures, because their dc electrons (2.156 eV/atom) are less than that of
=75%, mole fraction) and the strongest tendency to form hcp and bcc structures, because their dc electrons (2.156 eV/atom) are less than that of ![]() atoms, and would rather be in the eg state. The
atoms, and would rather be in the eg state. The ![]() atoms have the least concentration (
atoms have the least concentration (![]() =25%) and the weakest tendency to form fcc structure, because their sc electrons (1.32 electrons/atom) are less than that of
=25%) and the weakest tendency to form fcc structure, because their sc electrons (1.32 electrons/atom) are less than that of ![]() atoms. Therefore the
atoms. Therefore the ![]() atoms play the leading role in forming hcp lattice for D019- Ti3Al compound.
atoms play the leading role in forming hcp lattice for D019- Ti3Al compound.
4.2 Relationship of lattice stability with atomic energies and concentration for Ti3Al type alloys
The atomic cohesive energies and heat of formation of the hcp and fcc Ti3Al compounds are as follows:
ε(hcp Ti3Al)=-(0.75![]() +0.25
+0.25![]() )=4.810 8 eV/atom (5)
)=4.810 8 eV/atom (5)
ε(fcc Ti3Al)=-(0.75![]() +0.25
+0.25![]() )=4.795 2 eV/atom (6)
)=4.795 2 eV/atom (6)
?ε=ε(hcp Ti3Al)- ε(fcc Ti3Al)=0.015 2 eV/atom (7)
![]() (8)
(8)
![]() (9)
(9)
The ε(hcp Ti3Al) is slightly larger than ε(fcc Ti3Al), so the hcp Ti3Al compound is more stable than the fcc Ti3Al compound. The calculated heat of formation of the ?H(hcp Ti3Al) is smaller than its experimental values (-0.27 and -0.29 eV/atom)[14].
From the compositional variation of cohesive energies of the hcp Ti3Al, fcc Ti3Al, fcc TiAl and fcc TiAl3 type ordered TixAl(1-x) alloys (see Fig.3), it can be known that the hcp Ti3Al type ordered TixAl(1-x) alloys are more stable than other ordered alloys in the range of 0-40% Al (mole fraction).
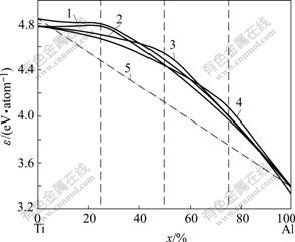
Fig.3 Atomic cohesive energies of hcp Ti3Al(1), fcc Ti3Al(2), fcc TiAl(3) and fcc TiAl3(4) type ordered TixAl(1-x) alloys and mixed solution [hcp Ti+fcc Al](5)
The calculated energy difference between hcp and fcc Ti3Al compounds is very small, which makes it feasible to stabilize the L12 phase by ternary alloy additions. Considering electronic structure and that the ![]() atoms play the leading role in forming hcp lattice for D019 Ti3Al compound, these ternary element additions should occupy the Ti-lattice points, and have much more dc-electrons than Ti-atoms.
atoms play the leading role in forming hcp lattice for D019 Ti3Al compound, these ternary element additions should occupy the Ti-lattice points, and have much more dc-electrons than Ti-atoms.
4.3 Relationship of lattice constants and concentra- tion of hcp Ti3Al type alloys
The average atomic volumes of several ordered TixAl(1-x) alloys with hcpTi3Al type are listed in Table 3. Because we still can not establish the function between the ratio c/a and concentration in theory for the ordered TixAl(1-x) alloys with hcp Ti3Al type, we have drawn the polynomial equation of the c/a—xAl curve from the experimental data of ratio c/a:
c/a=1.586 4+0.131 7xAl-0.085 07![]() (10)
(10)
The lattice constants of several hcp Ti3Al type TixAl(1-x) alloys are listed in Table 3. The lattice constants of the hcp Ti3Al compound are a=0.287 2 nm and c=0.456 4 nm, c/a=1.614, which are in good agreement with experimental values (a=0.287 5 nm, c=0.460 nm)[15].
5 Conclusions
1) It has been proved that the compositional variations of the electronic structure, atomic potential energies, atomic volumes, lattice constants and cohesive energies of the ordered hcp Ti3Al type alloys can be obtained from the basic information of sequences of Ti and Al characteristic atoms and corresponding characteristic crystals in the Ti-Al hcp lattice system.
2) The hcp Ti3Al compound is formed by ![]() and
and ![]() atoms. The electronic structure is 0.75[Ar](3dn)0.573? (3dc)2.168 5(4sc)0.972(4sf)0.309 3+0.25[Ne](3sc)1.32(3pc)1.19 (3sf)0.49. The calculated lattice constants and heat of formation of the hcp Ti3Al compound are in good agreement with experimental values.
atoms. The electronic structure is 0.75[Ar](3dn)0.573? (3dc)2.168 5(4sc)0.972(4sf)0.309 3+0.25[Ne](3sc)1.32(3pc)1.19 (3sf)0.49. The calculated lattice constants and heat of formation of the hcp Ti3Al compound are in good agreement with experimental values.
3) The factors of controlling crystalline structure are electronic structure, atomic potential energies and atomic concentration. Comparing ![]() and
and ![]() atoms in the hcp Ti3Al compound with the
atoms in the hcp Ti3Al compound with the ![]() and
and ![]() atoms in the fcc Ti3Al compound, it has been found that the
atoms in the fcc Ti3Al compound, it has been found that the ![]() atom has the least dc-electrons and the strongest tendency to form hcp and bcc structures, and the
atom has the least dc-electrons and the strongest tendency to form hcp and bcc structures, and the ![]() atom has the least sc-electrons and the weakest tendency to form fcc structure. Therefore, the
atom has the least sc-electrons and the weakest tendency to form fcc structure. Therefore, the ![]() atoms play the leading role in forming hcp Ti3Al compound.
atoms play the leading role in forming hcp Ti3Al compound.
4) The calculated cohesive energy of the hcp Ti3Al compound is slightly bigger than that of the fcc Ti3Al.This is a good sign that makes it feasible to stabilized L12 structure of the hcp Ti3Al compound by ternary element. The new element should have more dc-electrons than Ti-metal and occupy at the Ti-lattice points.
References
[1] THOMAS M, VASSEL A, VEYSSIERE P. Dissociation of superdislocations in the intermetallic compound Ti3Al [J].Scr Metall, 1987, 21: 501-506.
[2] FLEISCHER R L, DIMIDUK D M, LIPSITT H A. Electronic structure, cohesive properties, and phase stability of Ti3Al and Ni3Al [J]. Annu Rev Mater Sci, 1999, 19: 231-239.
[3] YAMAGUCHI M, UMAKASHI Y. In-situ TEM study of fracture mechanisms of poly synthetically twinned(PST) crystals of TiAl alloys [J]. Prog Mater Sci, 1997, 34: 1-9.
[4] CHUBB S R, PAPACONSTANTOPOULOS D A, KLEIN B M. First-principles study of L10 Ti-Al and V-Al alloys [J]. Phys Rev B, 1998, B38: 12120-12124.
[5] HONG T, WATSON-YANG T J, FREEMAN A J, OGUCHI T, XU J H. Crystal structure, phase stability, and electronic structure of Ti-Al intermetallics: TiAl3 [J]. Phys Rev B, 1990, B41: 12462-12467.
[6] HONG T, WATSON-YANG T J, FREEMAN A J. Crystal structure, phase stability and electronic structure of Ti-Al Intermetallic: Ti3Al [J]. Phys Rev B, 1991, B43: 1940-1947.
[7] ZHANG F, HUANG W, CHANG Y A. Equivalence of the generalized bond-energy model, the wagner-schottky-type model and the compound-energy model for ordered phases [J]. Calphad, 1997, 21: 337-348.
[8] ASTA M, DE FONTAINE D. First-principles study of phase stability of Ti-Al intermetallic compounds [J]. J Mater Res, 1993, 8: 2554-2569.
[9] XIE You-qing. The framework of metallic materials systematic science [J]. Mater Rev, 2001, 15(4): 12-15.
[10] XIE Y Q, PENG K, LIU X B. Influences of xTi/xAl on atomic states, lattice constants and potential energy planes of ordered FCC TiAl type alloys [J]. Physica B, 2004, 344: 5-20.
[11] XIE Y Q, LIU X B, PENG K. Atomic states, potential energies, volumes, brittleness and phase stability of ordered FCC TiAl3 type alloys [J]. Physica B, 2004, 353: 15-33.
[12] XIE Y Q, PENG H J, LIU X B. Atomic states, potential energies, volumes, brittleness and phase stability of ordered FCC Ti3Al type alloys [J]. Physica B, 2005, 362: 1-17.
[13] XIE Y Q, TAO H J, PENG H J. Atomic states, potential energies, volumes, brittleness and phase stability of ordered FCC TiAl2 type alloys [J]. Physica B, 2005, 366: 17-37.
[14] BRANDES E A. Smithells metals reference book [M]. London: Butterworths Press, 1983: 275.
[15] PEARSON W B. A handbook of lattice spacings and structures of metals and alloys [M]. New York: Pergamon, 1958: 103.
Foundation item: Project(50471058) supported by the National Natural Science Foundation of China; Project(06FJ3133) supported by the Natural Science Foundation of Hunan Province, China
Corresponding author: PENG Hong-jian; Tel: +86-731-8879287; E-mail: phj108@163.com


