Trans. Nonferrous Met. Soc. China 24(2014) 1659-1665
Effect of immersion Ni plating on interface microstructure and mechanical properties of Al/Cu bimetal
Jia-lei ZHAO, Jin-chuan JIE, Fei CHEN, Hang CHEN, Ting-ju LI, Zhi-qiang CAO
School of Materials Science and Engineering, Dalian University of Technology, Dalian 116024, China
Received 9 July 2013; accepted 3 December 2013
Abstract:
A nickel-based coating was deposited on the pure Al substrate by immersion plating, and the Al/Cu bimetals were prepared by diffusion bonding in the temperature range of 450-550 °C. The interface microstructure and fracture surface of Al/Cu joints were studied by scanning electron microscopy (SEM) and X-ray diffraction (XRD). The mechanical properties of the Al/Cu bimetals were measured by tensile shear and microhardness tests. The results show that the Ni interlayer can effectively eliminate the formation of Al-Cu intermetallic compounds. The Al/Ni interface consists of the Al3Ni and Al3Ni2 phases, while it is Ni-Cu solid solution at the Ni/Cu interface. The tensile shear strength of the joints is improved by the addition of Ni interlayer. The joint with Ni interlayer annealed at 500 °C exhibits a maximum value of tensile shear strength of 34.7 MPa.
Key words:
Al/Cu bimetal; immersion Ni plating; interface; diffusion bonding; intermetallics;
1 Introduction
Aluminum and its alloys are widely used for structure components in automobiles and aerospace because of their low density, and excellent corrosion resistance; while copper and its alloys have extraordinarily high level of electrical and thermal conductivity [1-5]. Recently, the joining between dissimilar materials has experienced rapid development due to their unique advantages compared to two raw metals in a single composition, among them, Al/Cu bimetals have received much attention because of the excellent performance. For example, compared to single copper alloy, Al/Cu bimetal offers a 40%-60% reduction in mass for equivalent conductivity and is 30%-40% less expensive [6,7]. So, they are widely used for electromagnetic winding line, shielding line, conductive strips, and armored cables [7-9].
Diffusion bonding is an advanced solid-bonded welding process which can join almost all materials with compatible metallurgical properties and avoid many defects such as crack, distortion and segregation [10]. However, the formation of complex Al-Cu intermetallic compounds (IMCs), such as Al2Cu, AlCu, Al3Cu4 and Al4Cu9 [8,11], at the interface of Al and Cu, is still a major problem in diffusion-bonded Al/Cu bimetals. Similar problems also exist in the other dissimilar bimetals and an intermediate material with stable physical and chemical performances is effective to control the formation of intermetallics. Recently, considerable works have been performed to study the effect of Ni interlayer on diffusion bonded Mg/Al [12,13], Mo/Cu [14], SS/Ti [15], etc, and the results indicated that the Ni interlayer can improve the mechanical properties of dissimilar bimetals. As Ni possesses substantial solid solubility in Al and Cu, it is considered to be a constructive interlayer in diffusion-bonded Al/Cu bimetals.
However, few researches have been conducted to study the effect of Ni interlayer on the interfacial structure and mechanical properties of Al/Cu bimetal, especially the effect of immersion Ni coating. Immersion Ni plating is the deposition of a Ni coating on a base metal from a nickel-based solution by means of chemical replacement, which shows excellent economy and simplicity [16]. In the present study, the Ni interlayer was prefabricated onto the cleaned aluminum surface by immersion plating, and diffusion-bonded Al/Cu joint with Ni interlayer was investigated.
2 Experimental
Pure Al (≥99.7%) and pure Cu (≥99.9%) were used as the base materials in the diffusion-bonding experiments. The samples were cut into cylinders with a diameter of 21.5 mm and a height of 6 mm.
Rigorous pre-treatment of the substrates was required to remove the contaminant and oxide. The surfaces of Al and Cu substrates were mechanically ground with emery papers up to 1500# to ensure similar surface roughness. In order to clean the contact surfaces, the pure Al and Cu were etched in 10% (mass fraction) NaOH and 15% (volume fraction) HCl solutions, respectively, then ultrasonically cleaned in ethanol for 1 min. After water rinsed for 1-2 min, the Cu billet was dried in air and the Al billet was placed in the immersion bath containing 400 g/L NiCl2, 50 g/L H3BO4 and 25 mL/L HF. The time interval between the pre-treatment and the immersion plating must be small to avoid the oxidation of the substrate and to insure adhesion of the coating layer. The plating process was carried out at room temperature for 10 min under supersonic agitation.
The manufacturing of Al/Cu bimetal was carried out in an annealing furnace. The compression load of the sample was imposed by a steel fixture and the details of the experimental setup were shown in a previous work [17]. The Al/Cu joints with Ni interlayer were heat- treated at 450 °C, 500 °C, and 550 °C, respectively. To investigate the effect of Ni interlayer, the Al-Cu direct joint was heat-treated at 450 °C as a comparison. The heating rate was 10 °C/min and the temperature deviation of the annealing furnace was ±2 °C. All the joints were annealed at the constant temperature for 30 min and then cooled to room temperature in the furnace.
To identify the microstructure and phase composition of the interfacial zone of Al/Cu bimetals, the transverse section of diffusion-bonded samples was mechanically polished and scanning electron microscope (Supra 55) equipped with an energy dispersive X-ray (EDX) spectrometer was used. The phase constitution of the fracture surface was identified by PANALYTICAL- EMPYREAN X-ray diffractometer (XRD). The Vickers hardness along the thickness of intermediate phases was measured by an Everone MH-60 microhardness tester with a load of 10 g and dwell time of 15 s. The average hardness was calculated from three indentations. The tensile shear test was conducted on a DNS-100 electronic strength tester at a velocity of 1.0 mm/min. The strength is taken as:
τ=F/BL (1)
where τ is the tensile shear strength, F is the maximum shear load, B and L are the width and length of lapped surface, respectively. In the present study, B=(5.0±0.05) mm, L=(16.0±0.05) mm.
3 Results and discussion
The surface morphology and elemental composition of the Ni coating are shown in Fig. 1. It can be seen from Fig. 1(a) that the coating surface exhibits a compact cell-shaped structure and the Ni cells are composed of numerous white ellipsoidal particles. The EDX result reveals that the primary composition of the coating is Ni. The transverse section of Al substrate with Ni coating was mechanically ground to estimate the thickness of the coating which is in the range of 8-12 μm, as shown in Fig. 1(b).
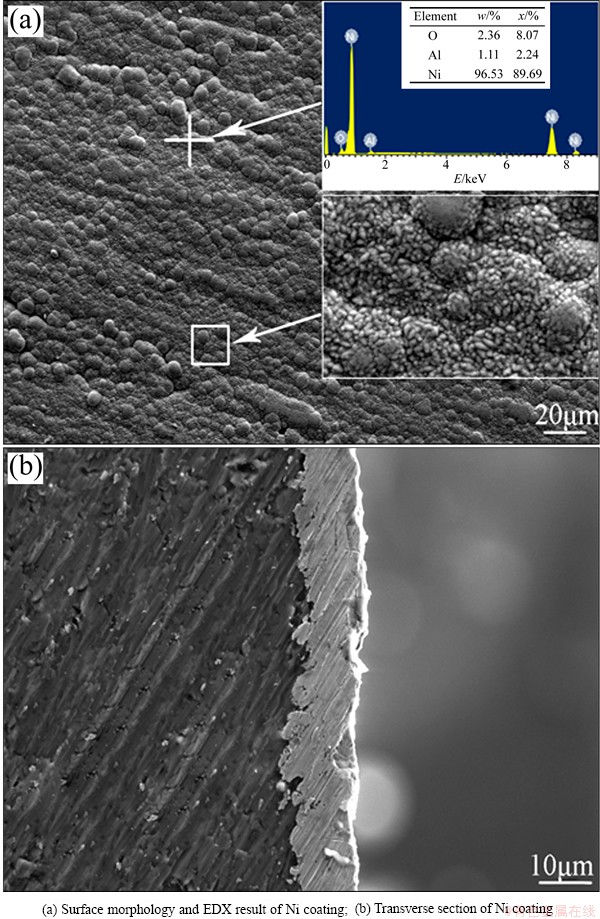
Fig. 1 SEM images of Ni coating deposited on Al substrate for 10 min
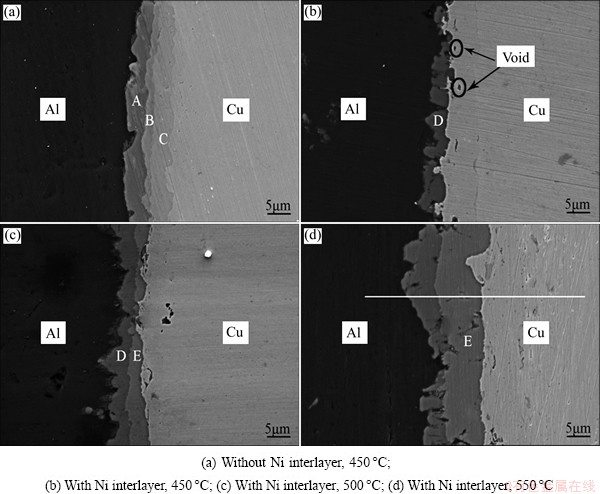
Fig. 2 Secondary electron SEM images at Al/Cu interface heat treated at different temperatures
Table 1 EDX results taken from different zones as denoted in Fig. 2
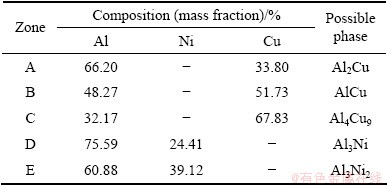
Figure 2 illustrates the interface microstructures of the Al/Cu couples without and with Ni interlayer heat treated at different temperatures. The results of EDX analysis conducted on zones A, B, C, D and E in Fig. 2 are summarized in Table 1. Figure 2(a) shows the interface microstructure of the Al/Cu direct joint heat-treated at 450 °C for 30 min, and three distinct reaction layers can be clearly observed. The zones A, B, and C are identified as Al2Cu, AlCu, and Al4Cu9, respectively, which is consistent with the previous reports [18,19]. The SEM image of the Al/Cu joint with Ni interlayer heat-treated at 450 °C is shown in Fig. 2(b). It can be observed that a discontinuous phase (marked D), which is identified to be Al3Ni, is formed at the interface of Al/Ni. In addition, there exist some voids on the Ni interlayer due to the difference in the atomic fluxes across the Al/Ni interface during diffusion-bonding process. When the joint is annealed at 500 °C for 30 min, the Al3Ni layer exhibits good continuity and the Al3Ni2 phase (marked E) is formed between the Al3Ni and Ni layers, as shown in Fig. 2(c). The formation of Al3Ni and Al3Ni2 phases can be described according to the following equations [20]:
3Al+Ni→Al3Ni+1083.6 kJ/mol (2)
Al3Ni+Ni→Al3Ni2+378.1 kJ/mol (3)
With the elevation of annealing temperature, both Al3Ni and Al3Ni2 intermediate layers become thick, especially for the Al3Ni2 layer (Fig. 2(d)). During annealing process, the existence of Al3Ni2 layer restrains the diffusion of Ni atoms to Al substrate and a large proportion of Ni atoms are exhausted for the growth of Al3Ni2 layer, which leads to a much smaller thickness of Al3Ni layer [21]. It should be noted that the thickness of Al-Ni intermediate phases is not uniform because of the cell structure of the Ni coating, furthermore, the existence of oxide can prevent the contact of the surfaces at the beginning of joint formation [22].
In order to further investigate the phases between the Al and Cu substrates, the composition distribution of the Al/Cu joint annealed at 550 °C for 30 min was measured by line scanning. Figure 3 shows the distribution of major elements (Al, Ni and Cu) along the white line in Fig. 2(d). It can be seen clearly that the interdiffusion between Al and Cu substrates is impeded by the Ni interlayer. The Al-Ni IMCs (Al3Ni and Al3Ni2) are formed due to the interdiffusion between Al and Ni, and the thicknesses of Al3Ni and Al3Ni2 phases are about 6 μm and 9 μm, respectively. The zones F and G present a gradient distribution of Ni and Cu, which are considered to be Ni(Cu) and Cu(Ni) solid solution, respectively. In addition, the width of zone F is much thicker than that of zone G, which should be ascribed to the difference in diffusion coefficient of Cu in Ni (DCu-Ni) and Ni in Cu (DNi-Cu) as follows:
DCu-Ni=1.01×10-7exp[-149/(RT)] m2/s [23] (4)
DNi-Cu=7.0×10-5exp[-225/(RT)] m2/s [24] (5)
where R is the gas constant (8.314 J/(mol·K)) and T is the thermodynamic temperature. At 550oC, the diffusion coefficient of Cu in Ni (DCu-Ni=4.0×10-17 m2/s) is greater than that of Ni in Cu (DNi-Cu=3.7×10-19 m2/s), so the amount of Cu diffusion into Ni is larger than that of Ni diffusion into Cu.
To determine the hardness variation between Al and Cu substrates, microhardness profiles were measured across the diffusion-bonded joint with Ni interlayer at 550 °C for 30 min, and the results are shown in Fig. 4. It can be seen that the Al and Cu base materials have average hardness of HV 42 and HV 85, respectively. The interfacial area generally possesses higher hardness than the Al or Cu substrate. The reaction layer exhibits a maximum value of HV 720, due to the formation of Al-Ni intermetallics. The Ni-Cu interface reaches a microhardness of HV 280 because of the existence of Ni-Cu solid solution layer.
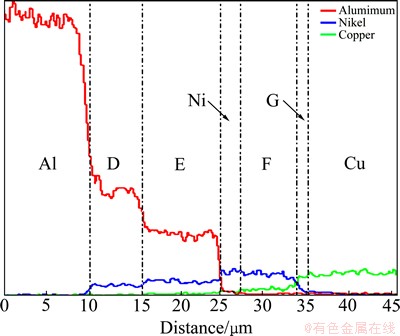
Fig. 3 Element distribution across Al/Cu joint annealed at 550 °C for 30 min
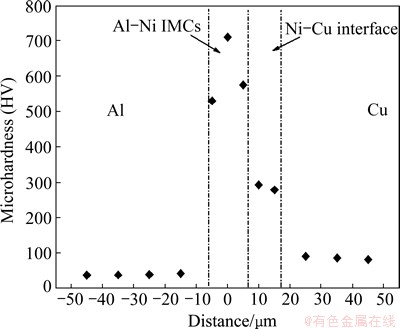
Fig. 4 Microhardness profiles of joint with Ni interlayer heat treated at 550 °C for 30 min
The tensile shear test was performed to evaluate the influence of Ni interlayer on the bonding strength of the Al/Cu joints. The tensile shear load and strength were averaged from three values and the results are shown in Table 2. It can be seen that the tensile shear strength of the Al/Cu direct joint annealed at 450 °C for 30 min is 14.3 MPa. With a Ni interlayer, the strength increases to 25.9 MPa under the same annealing condition. When the joint is annealed at 500 °C for 30 min, the strength of Al/Cu joint with Ni interlayer reaches 34.7 MPa which is greater than that annealed at 450 °C. However, the strength of Al/Cu joint with Ni interlayer annealed at 550 °C drops severely to 12.4 MPa, due to the high volume fraction of Al3Ni and Al3Ni2 brittle compounds.
Table 2 Tensile shear load and strength of Al-Cu joints
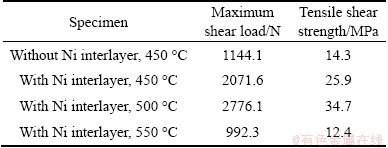
It was reported that the dissimilar bimetallic joints rapidly lost its mechanical integrity when the total width of intermetallic layers exceeded 5 μm [12]. During direct bonding of Al/Cu, a large number of Al-Cu intermetallic compounds inevitably form at the interface, which greatly reduces the mechanical properties of the joint. According to Ref. [25], the parabolic growth constant k of Al-Cu intermetallics is 4.11×10-10m2/s at 700 °C, while it is 6.59×10-15m2/s for Al-Ni intermetallics at 720 °C [21]. It means that Al-Cu intermetallics grow much faster than Al-Ni intermetallics. Therefore, under the same annealing conditions for Al-Cu direct joint, the Ni interlayer can effectively reduce the thickness of brittle intermetallics. As a result, the mechanical property of the joint is improved. However, when a Ni interlayer is added between the Al and Cu substrates, the strength of the bonding region depends not only on the quality of the Al-Ni interface but also on the strength of Ni-Cu solid solution. At 450 °C, although the bonding between Al substrate and Ni interlayer is good, the element diffusion of Ni and Cu is not enough to form a high-strength solid solution which results in the poor performance at this temperature. At 500 °C, there is a small amount of increase in the thickness of Al-Ni compounds, but the bonding strength is still high. Furthermore, a high- strength Ni-Cu solid solution can be formed at this temperature. But when the annealing temperature reaches 550 °C, a mass of Al-Ni intermetallics can be formed at the Al-Ni interface, which is detrimental to the mechanical property of the joint. Based on the analysis above, a conclusion can be drawn that the Ni interlayer is useful to improve the mechanical properties of Al/Cu bimetal; in addition, joint with Ni interlayer annealed at 500 °C exhibits high performance due to the good bonding of Al-Ni and Ni-Cu.
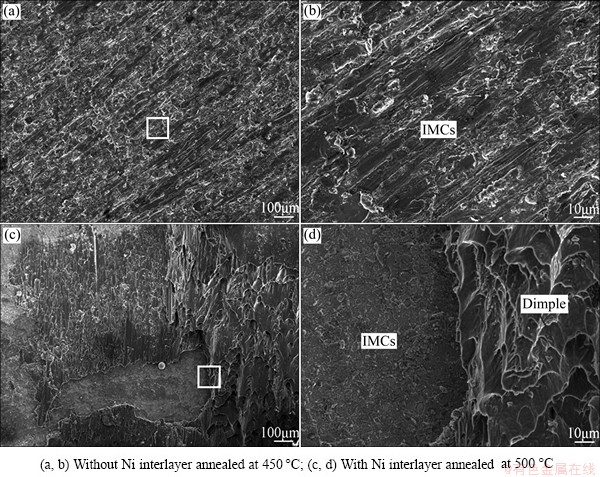
Fig. 5 Fracture surfaces of Al/Cu joint on Cu side
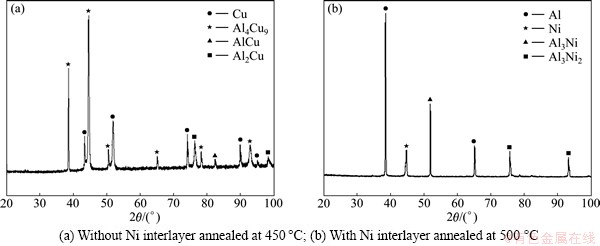
Fig. 6 XRD patterns of fracture interface
To identify the fracture mechanism of the Al/Cu joint prepared under different conditions, the morphology and the phase constitution of the fracture surfaces on the Cu side were investigated, and the results are shown in Fig. 5 and Fig. 6, respectively. For Al/Cu direct joint, the fracture surface is full of Al-Cu intermetallics, as shown in Fig. 6(a), indicating that the fracture takes place at the Al-Cu interface. It can be seen from Figs. 5(a) and (b) that the ruptured compounds exhibit a specific tropism along the tensile shear direction with some cleavage facets and white strip torn edges on the surfaces, which indicates the rupture mechanism of the joint is quasi-cleavage fracture. For Al/Cu joint with Ni interlayer annealed at 500 °C (Figs. 5(c), (d) and Fig. 6(b)), a large area of Al substrate can be observed on the fracture surface which is indicative of ductile fracture with some dimples. It implies that the tensile shear strength in these areas is higher than that of the Al substrate, which is mainly responsible for the increasing of the tensile shear strength. However, there is partial brittle fracture in the interfacial region, which should be related to the non-uniform thickness of the reaction layer. In regions with thicker reaction layer, the effect of inherent brittleness of the intermetallic compounds is more prominent. In addition, the residual and internal stresses which result from diffusion are easy to concentrate in these regions, which facilitates the growth of cavity and crack [26]. As a result, the bonding strength is lower and fracture is apt to occur at the interface.
It can be convinced from the analysis above that there is a strong relationship between the fracture of the Al/Cu joints and the thickness of the brittle intermetallics. When the reaction layer is thin, the fracture occurs on the Al side, while the fracture occurs at the interface under the condition that the thickness of the reaction layer is above the critical value. The addition of a Ni interlayer can effectively reduce the thickness of brittle compounds and then change the fracture path and mechanism, as a consequence, the bonding strength is increased. However, additional work should be done to make out the growth behavior of Al-Ni intermetallics and the critical value of the thickness to further improve the mechanical property of Al-Cu joints.
4 Conclusions
1) The Ni interlayer by immersion plating can effectively restrain the formation of Al-Cu intermetallic compounds, but results in the formation of Al-Ni compounds. However, the growth rate of Al-Ni compounds is much smaller than that of Al-Cu compounds under the same annealing condition. With the elevation of temperature, the Al3Ni reaction layer is formed at the Al/Ni interface first, and then the Al3Ni2 reaction layer appears. The interfacial zone consists of Al3Ni, Al3Ni2, Ni interlayer, Ni(Cu) solid solution and Cu(Ni) solid solution from the Al side to Cu side.
2) The strength of the Al-Cu joint with Ni interlayer annealed at 500 °C is 34.7 MPa and it is higher than that of the direct joint of Al and Cu, which can be ascribed to the formation of Ni-Cu solid solution and the small thickness of Al-Ni intermetallics. The fracture of Al-Cu direct joint takes place at the interface completely, while for joint with Ni interlayer annealed at 500 °C, the fracture partially takes place at the Al substrate.
References
[1] SARSILMAZ F. Weldability characteristics of dissimilar Al/Cu friction stir weld joints [J]. Materials Testing, 2012, 54: 85-91.
[2] LEE S M, LEE M G, LEE S P, LEE G A, KIM Y B, LEE J S, BAR D S. Effect of bonding interface on delamination behavior of drawn Cu/Al bar clad material [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: s645-s649.
[3] SASAKE T T, MORRIS R A, THOMPSON G B, SYARIF Y, FOX D. Formation of ultra-fine copper grains in copper-clad aluminum wire [J]. Scripta Materialia, 2010, 63: 488-491.
[4] XIA C Z, LI Y J, PUCHKOV U A, GERASIMOV S A, WANG J. Microstructure and phase constitution near the interface of Cu/Al vacuum brazing using Al-Si filler metal [J]. Vacuum, 2008, 82: 799-804.
[5] XIA C Z, LI Y J, WANG J, MA H J. Microstructure and phase constitution near interface of Cu/Al vacuum brazing [J]. Materials Science and Technology, 2007, 23: 815-818.
[6] ESLAMI P, KARIMI-TAHERI A. An investigation on diffusion of aluminum to copper using equal channel angular extrusion process [J]. Materials Letters, 2011, 65: 1862-1864.
[7] RHEE K Y, HAN W Y, PARK H J, KIM S S. Fabrication of aluminum/copper clad composite using hot hydrostatic extrusion process and its material characteristics [J]. Materials Science and Engineering A, 2004, 384: 70-76.
[8] CHEN C Y, HWANG W S. Effect of annealing on the interfacial structure of aluminum-copper joints [J]. Materials Transactions, 2007, 48: 1938-1947.
[9] ABDOLLAH-ZADEH A, SAEID T, SAZGARI B. Microstructural and mechanical properties of friction stir welded aluminum/copper lap joints [J]. Journal of Alloys and Compounds, 2008, 460: 535-538.
[10] SABETGHADAM H, ZAREI-HANZAKI A, ARAEE A. Diffusion bonding of 410 stainless steel to copper using a nickel interlayer [J]. Materials Characterization, 2010, 61: 626-634.
[11] ABBASI M, KARIMI TAHERI A, SALEHI M T. Growth rate of intermetallic compounds in Al/Cu bimetal produced by cold roll welding process [J]. Journal of Alloys and Compounds, 2001, 319: 233-241
[12] ZHANG J, LUO G Q, WANG Y Y, XIAO Y, SHEN Q, ZHANG L M. Effect of Al thin film and Ni foil interlayer on diffusion bonded Mg-Al dissimilar joints [J]. Journal of Alloys and Compounds, 2013, 556: 139-142.
[13] ZHANG J, LUO G Q, WANG Y Y, SHEN Q, ZHANG L M. An investigation on diffusion bonding of aluminum and magnesium using a Ni interlayer [J]. Materials Letters, 2012, 83: 189-191.
[14] LUO G Q, ZHANG J, LI M J, WEI Q Q, SHEN Q, ZHANG L M. Interfacial microstructure and mechanical strength of 93W/Ta diffusion-bonded joints with Ni interlayer [J]. Metallurgical and Materials Transactions A, 2013, 44: 602-605.
[15] KUNDU S, CHATTERJEE S. Characterization of diffusion bonded joint between titanium and 304 stainless steel using a Ni interlayer [J]. Materials Characterization, 2008, 59: 631-637.
[16] HIRSCH S, ROSENSTEIN C. Immersion plating [J]. Metal Finishing, 2000, 98: 436-439.
[17] MIRJALILI M, SOLTANIEH M, MATSUURA K, OHNO M. On the kinetics of TiAl3 intermetallic layer formation in the titanium and aluminum diffusion couple [J]. Intermetallics, 2013, 32: 297-302.
[18] LEE K S, KWON Y N. Solid-state bonding between Al and Cu by vacuum pressing [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 341-346.
[19] HENESS G, WUHRER R, YEUNG W Y. Interfacial strength development of roll-bonded aluminium/ copper metal laminates [J]. Materials Science and Engineering A , 2008, 483-484: 740-742.
[20] KE L M, HUANG C P, XING L, HUANG K H. Al-Ni intermetallic composites produced in situ by friction stir processing [J]. Journal of Alloys and Compounds, 2010, 503: 494-499.
[21]  G A, SOMMADOSSI S, GUST W, MITTEMEIJER E J, ZIEBA P. Phase characterization of diffusion soldered Ni/Al/Ni interconnections [J]. Interface Science, 2002, 10: 13-19.
G A, SOMMADOSSI S, GUST W, MITTEMEIJER E J, ZIEBA P. Phase characterization of diffusion soldered Ni/Al/Ni interconnections [J]. Interface Science, 2002, 10: 13-19.
[22] GUO Y J, LIU G W, JIN H Y, SHI Z Q, QIAO G J. Intermetallic phase formation in diffusion-bonded Cu/Al laminates [J]. Journal of Materials Science, 2011, 46: 2467-2473.
[23] ANAND M S, MURARKA S P, AGARWALA R P. Diffusion of copper in nickel and aluminum [J]. Journal of Applied Physics, 1965, 36: 3860-3862.
[24] BERNARDINI J, CABANE J. Ruthenium and nickel pipe diffusion in copper [J]. Acta Metallurgica, 1973, 21: 1571-1578.
[25] TANAKA Y, KAJIHARA M, WATANABE Y. Growth behavior of compound layers during reactive diffusion between solid Cu and liquid Al [J]. Materials Science and Engineering A, 2007, 445-446: 355-363
[26] HANG C J, WANG C Q, MAYER M, TIAN Y H, ZHOU Y, WANG H H. Growth behavior of Cu/Al intermetallic compounds and cracks in copper ball bonds during isothermal aging [J]. Microelectronics Reliability, 2008, 48: 416-424.
浸镀Ni对Al/Cu双金属材料界面显微组织及力学性能的影响
赵佳蕾,接金川,陈 飞,陈 航,李廷举,曹志强
大连理工大学 材料科学与工程学院,大连 116024
摘 要:采用浸镀的方法在纯铝基体上浸镀镍基镀层,然后在450~550 °C温度范围内用扩散复合的方法制备Al/Cu双金属材料。用扫描电子显微镜(SEM)和X射线衍射仪(XRD)分别对Al/Cu结合体的界面显微组织以及断裂表面进行表征。用拉伸剪切测试及显微硬度测试对Al/Cu双金属材料的力学性能进行测量。结果表明,Ni中间层可以有效地消除Al-Cu金属间化合物的形成。Al/Ni界面由Al3Ni 和Al3Ni2两相组成,而在Ni/Cu界面处则是Ni-Cu固溶体。Ni中间层的加入提高了复层材料的拉伸剪切强度。在500 °C制备的添加Ni中间层的试样表现出最大的拉伸剪切值,为34.7 MPa。
关键词:Al/Cu双金属材料;浸镀Ni;界面;扩散连接;金属间化合物
(Edited by Sai-qian YUAN)
Foundation item: Projects (51274054, 51375070, 51271042) supported by the National Natural Science Foundation of China; Project (2013M530913) supported by the China Postdoctoral Science Foundation
Corresponding author: Zhi-qiang CAO; Tel: +86-411-84706169; E-mail: caozq@dlut.edu.cn
DOI: 10.1016/S1003-6326(14)63238-6
Abstract: A nickel-based coating was deposited on the pure Al substrate by immersion plating, and the Al/Cu bimetals were prepared by diffusion bonding in the temperature range of 450-550 °C. The interface microstructure and fracture surface of Al/Cu joints were studied by scanning electron microscopy (SEM) and X-ray diffraction (XRD). The mechanical properties of the Al/Cu bimetals were measured by tensile shear and microhardness tests. The results show that the Ni interlayer can effectively eliminate the formation of Al-Cu intermetallic compounds. The Al/Ni interface consists of the Al3Ni and Al3Ni2 phases, while it is Ni-Cu solid solution at the Ni/Cu interface. The tensile shear strength of the joints is improved by the addition of Ni interlayer. The joint with Ni interlayer annealed at 500 °C exhibits a maximum value of tensile shear strength of 34.7 MPa.


