Article ID: 1003-6326(2005)03-0680-06
Photocatalytic degradation of dimethomorph on nanometer titanium dioxide by silver depositing in aqueous suspension
YAN Jian-hui(阎建辉)1, 2, HUANG Ke-long(黄可龙)1,
LIU Su-qin(刘素琴)1, ZENG Heng-zhi(曾恒志)2
(1. School of Chemistry and Chemical Engineering,
Central South University, Changsha 410083, China;
2. Department of Chemistry and Chemical Engineering,
Hunan Institute of Science and Technology, Yueyang 414000, China)
Abstract:
A series of catalysts of nanometer TiO2 were prepared by silver depositing. The photocatalytic degradation of dimethomorph in an aqueous suspensions by silver depositing with nanometer titanium dioxide as catalyst was investigated by radiation of UV-light and sunlight. A pseudo-first-order kinetic model was used to describe the results. The effects of the dosage of catalyst, oxidant, pH and radiation source on degradation were examined. The experimental results show that the decomposed rate of DMM is 94% when the dosage of catalyst is 1.25g/L and the concentration of DMM is 100mg/L under the conditions of solution pH of 7, the air flow of 1.5L/min and shining for 5h by UV-light. When it is shined by sun-light under the same condition, the decomposed rate of DMM is 48%. The mechanism of decomposition was discussed based on the data by analysis of LC-MS.
Key words:
dimethomorph; photocatalytic degradation; nanometer TiO2; silver deposition CLC;
number: X730.1; O625 Document code: A
1 INTRODUCTION
The development of industrialization brings us serious pollutions, especially the water pollution, which is harmful to the health of people and the survive of creatures. How to solve this problem is extremely important. In recent years, people have used TiO2 to photocatalytically degrade the water pollutants such as surfactants[1], herbicides[2], insecticides[3] and aromatic organics[4]. Some effect has been gotten. Photocatalyst TiO2 has been widely considered perfect physical-chemistry material for environment remedy during past two decades[5]. In the state of suspended[6, 7] or fixed bed[8, 9], by using the low energy UV-light and anatase TiO2, these pollutants could be degraded and even wholly mineralized.
Dimethomorph(DMM), a kind of pesticide of cinnamic acid compounds derivative, has a little solubility in water and is dissoluble in normal organic solvent. It has specific effect on the germs of peronosporales, phytophthora, so it is a new systemic high-efficiency fungicide to prevent from and cure of the peronosporales germs disease. It also has high activity to benamidate fungicides resistance and sensing strains, and is widely used in vegetables and fruits[10], such as cucumber, litchi, sweet pepper and grape. There is no effective method to deal with the polluted water containing DMM. The normal way of solving this problem is dilution biochemistry, but it is high in multiple load running cost and causes secondary pollution. Though many studies have been done in treatment of pollutions by nano-TiO2, systematic investigation about the photocatalytic reduction of DMM was not reported by using nano-TiO2, especially the nano-TiO2 by Ag depositing. In this paper, nano-Ag/TiO2 was used for photocatalytic degradation of DMM in aqueous suspension. The optimum conditions were investigated, and the experimental data were provided for application research of the self-degrade “green” nano-pesticide.
2 EXPERIMENTAL
2.1 Chemicals and apparatus
Dimethomorph (provide by Hunan Research Institute of Chemical Industry, with chemical structure shown in Fig.1, in which Z and E occupy 59% and 41% respectively), Ti(SO4)2 (Shanghai Chemical Reagent Corporation, China Medicine Group), AgNO3 (Shanghai Chemical Reagent First Plant, China); 1, 2-dichloroethane(Tientsin Tianda Chemical Reagent Plant) and methanol (Tianjin Kermel Chemical Reagent Development Center) were all analytically pure. Secondarily distilled water was used.
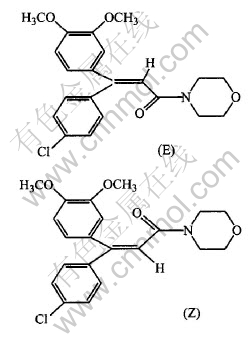
Fig.1 Chemical structure of dimethomorph
Type 1100 HPLC(US), Agilent 1100 series LC-MSD(US), pHS-29A Acidometer(Shanghai), heating and magnetic stirrer(Shanghai), G13T8, 10W medical sterilizing lamp(Hailin), GCD-300A generator of hydrogen(Beijing), 2D-2 high-speed multiple-oscillator(Jindan), SB3200-7 ultrasonic purifier(Shanghai), AA320 atomic absorption spectrometry(Shanghai), TGL-16G high speed centrifuge(Hunan) and ST-85 illuminometer(Beijing) were used.
2.2 Preparation of Ag/TiO2
16g TiO2 (calcined in 500℃ for 1h) which was prepared by hydrolysis-sol method[11] and 30mL AgNO3 ( concentration 0.01mol/L) were added into a tri-hole flask, and the total volume of reaction solution was 100mL by adding distilled water. The tri-hole flask was set in thermostatic water bath box. After 30min agitation at the temperature of 80℃, H2 was ventilated toward the reaction system with a rate of 330-340mL/min to reduce Ag+ for 1h. Then the suspension was centrifugally separated to obtain the light brown sample with loading Ag of 0.125%(molar fraction). Adding HCl without white deposition and using atomic absorption spectroscopy to detect the concentration of Ag+, and it was judged that Ag+ had almost reduced and deposited on the surface of the TiO2 particles. The configuration and granularity of Ag/TiO2 were the same as those of the original TiO2.
2.3 Experiment of photocatalytic degradation
A photoreactor consisted of a crock of jacket glass (12cm in diameter) where subcooling water was passed with a magnetic stirrer, and two 10W UV lamp. The lamp mainly provided light of wavelength of about 254nm. The distance between lamp and liquid level was 15cm, and the average light strength was 480lx. The whole reactor was placed in an opaque box. In experiment, the acquired catalyst was first weighed and added into water for ultrasonic separation for about 30min and then water and dimethomorph (l0g/L) were mixed to get a reaction mixture with certain concentration and total volume. It was quickly put into reactor and agitating in dark for 30min to get absorption equilibrium, then air was connected with flux of 1.5L/min. At the same time, 5mL samples were taken from the suspension and put into conical flask, then 5mL 1,2-dichloroethane was added for vibrated extraction for about 1h. Then it was separated by tundish of apart solution. Organic phase was used for determination of DMM. Fine days were chosen during September and November from 9am to 4pm in Yueyang, Hunan to do the experiment of sunshine reduction. The average luminous flux was 6.5×104lx. The experiment conditions and analytic methods were the same as those mentioned above.
2.4 Analytic method
The concentration during the degradation of DMM could be analyzed by HPLC (Type HP1100) and detector UV. The chromatographic condition was: chromatographic column ODS Hypersil stainless steel, specification 200mm×4.6mm in size, methanol/water 65∶35(volume ratio), flux 0.8mL/min and column temperature 26℃. Wavelength was 243nm, retention time for E was 9.21min and for Z was 10.58min. The main intermediate that decomposed from DMM could be detected by LC-MS and chromatographic column Zorbax Eclipse XDB-C8 in which specification was 150mm×4.6mm in size.
3 RESULTS AND DISCUSSION
3.1 Influencing factors of photocatalytic degradation
3.1.1 Dosage of photolycatalysis
Based on active appraisal on catalyst, TiO2 dried at 80℃ with deposited Ag of 0.125%(molar fraction) was used. When DMMs concentration was 100mg/L, reaction temperature was (26±2)℃ and solution was neutrality, five different catalysts from 0.5 to 1.5g/L were chosen for UV degradation. The DMMs decomposition rate varied with time is shown in Fig.2. There is an optimum value of catalyst concentration. As it is smaller than 1.25g/L, degradation rate is increased with increasing catalyst concentration. When it is more than 1.25g/L, degradation rate is decreased with increasing catalyst concentration in the same condition.
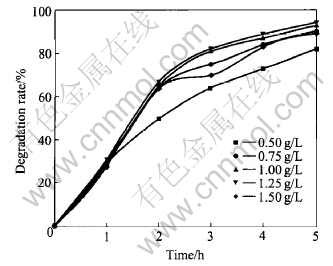
Fig.2 Effect of catalyst concentration on degradation rate
This is due to optical behavior of nano-particle separated in the solution. The nano-particles are dispersed in water to form sol, and generate Tyndall effect, in which the particle diameter is less than incident light wavelength. So UV-light can diffuse around the nano-particle and scatter. Based on Relly formula[12]:
I=24π3NV2/λ4(n21-n22)/(n21+n22)I0(1)
when incident light intensity I0, wavelength λ, volume of particles V and refractive ration of disperse phase (nano-particle ), disperse medium (water) are changeless, scatter intensity I is proportion to nano-particle number per unit volume N. Hence the higher the catalytic concentration and the more the particles per unit volume, the stronger the dispersion. In another word, UV-light that can be directly used is decreased and equalized to decrease the light strength. At the same time, high concentration suspension has a large effect on permeation of light. When the catalysts concentration is very low, the available active points of catalytst are less. So the active free radical·OH is less and the effect of degradation is not good.
3.1.2 pH of solution
Original pH of system not only affects the characteristic of degraded component but also affects the catalysts activity of Ag/TiO2. Because the pH value of solution affects the photochemistry of TiO2 and so does redox potential of degraded component[13], the experiments are carried out with original pH values of 3.0, 5.0, 7.0, 9.0 and 11.0(Fig.3). In the range of pH〈7, the lower the pH value, the larger the degraded rate. When pH>7, degraded rate becomes higher than that under the acidic condition before 1h, but will be lower than that under the acidic condition after 1h reaction.
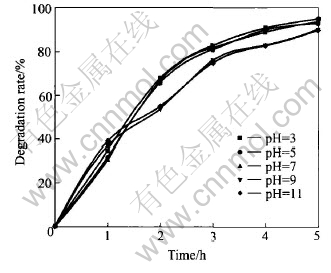
Fig.3 Effect of pH value on degradation rate
Although DMM is a neutral material, the charge density of atoms is different. The difference is dependent on not only the inbeing of atoms, but also the interaction between molecule and surface of catalyst. This will directly increase or reduce the photocatalytic degradation rate. The pH for zero charge of TiO2(P-25) surface is 6.3[14]. Namely when pH〈6.3, the surface of TiO2 will get positive charge. When pH>6.3, it will get negative charge. The balanceable process is
![]()
According to Ref.[15], the compounds as DMM have carbonyl, azyl, ether-oxide bone and halogen, which contain atoms O, N and halogen that have lone-pair electron. Their electronegativity is so strong that they appears negatively and is increased with the increase of pH of solution. Under the acidity condition, TiO2 with positive charge on the surface will be helpful to the absorption of DMM. While under the alkalescence condition, TiO2 with negative charge on the surface will not be helpful to the absorption of DMM. It is found out that absorption ability of molecule will directly affect the photocatalytic degradation rate.
Table 1 lists the pH values at different catalytic reaction times. From Table 1, it can be seen, whether the solution is acidic or alkalic, the final pH after photocatalytic degradation will be acidic. In another word, with the processing of reaction, the pH will go down. The decrease in pH is attributed to mineralized DMM or other organics into CO2 and congregated electron on nano-particle of Ag. While the reason for generating H+ is the oxidation of water by hole. The pH is departed from the theoretical adjusting values before reaction. Especially when pH=9, the actual value is far from theoretical value. This is mainly because of surface absorption equilibrium of catalytic (adsorbing H+ and OH-). In Table 1, the pH increases after reaction for 4h under acidic condition, because the catalysts surface needs to absorb more H+ to arise pH which is increased after mineralization of most organics. It is also found that pH values have a large effect when suspensoid is separated. It is confirmed that pH will affect aggregation of particles, as well as surface charge of catalysts.
Table 1 Change of pH value during reaction
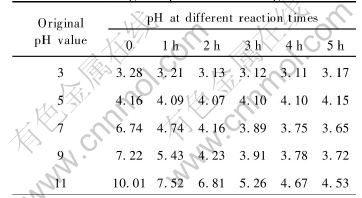
When pH=9 and 11, maybe under the high OH- concentration condition, α, β-unsaturated ketones ethylene linkage of DMM will make additive reaction take place easily. It breaks the conjugated system (Absorption peaks corresponding to position of HPLC are decreased ) of whole molecule. So, the degradation rate is high apparently. With the reaction going on, the photocatalytic reduction rate will be lower, because [OH-] concentration decreases. But under the acidic condition, the photocatalytic reduction rate will be higher or little changed because [H+] concentration always increases or is little changed.
3.1.3 Oxidant
Fig.4 shows the dependent curve of degradation rate of DMM on concentration of (NH4)2S2O8 while using Ag/TiO2 as catalysts and reaction for 1h. From Fig.4, it is known that degradation rate will be greatly improved by adding oxidant. Increase in the concentration of oxidant is of benefit to degradation rate. When 2.00mmol oxidant is added, namely the concentration is 5.0mmol/L, the degradation of DMM reaches 50%. (When equal H2O2 is added, the degradation rate reaches 41.8%). Under the same conditions, degradation rate is 31%. When the oxidants are added sequentially, the degradation rate of DMM is increased. Peroxide is called electron acceptor. As a good capture electron compound during the reaction, it can capture the photogenerated electrons of catalyst surface, prevent the recombination of electron and hole[16], and come into being more active ·OH. On the other hand, peroxide can be decomposed and generate a new active free radical[17]. In another word, by adding the peroxide, photon-efficiency will be improved and free radical will be increased.
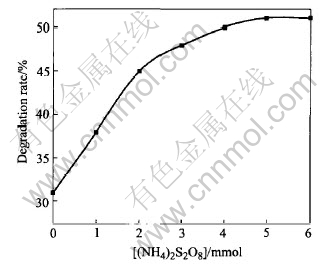
Fig.4 Effect of concentration of (NH4)2S2O8 on degradation rate(1h)
3.1.4 Total volume of suspensoid
Keeping other condition unchanged, we find that different volumes of suspensoid will bring about the largest effect on degradation rate of DMM (Fig.5). This is related to the fact that unit volume might accept different light strength and catalysts would accept different numbers of photons at the same time. So it will affect the number of photo-generated electrons and holes. Moreover, it will affect efficiency of degradation of DMM. Bhatkhande et al[18] think that photo-catalytic decomposition rate obeys Langmuir-Hinshelwood mechanism. The speed of reaction is not only the function of the catalysts stuff, the light strength, the reaction rate constant, but also the light illumed depth (the volume of solution or shape). They got an experience formula: K=3.81(1/V), and common instances of this law will be better followed.
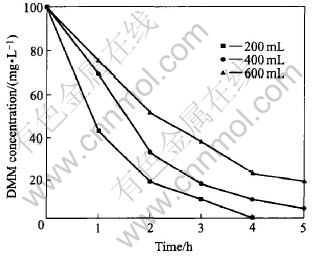
Fig.5 Effect of solution volume of reaction on degradation rate
3.2 Kinetics of degradation of DMM
Photocatalytic reaction kinetics of catalyst TiO2 obeys the Langmuir-Hinshelwood Equation[18]:
υ=k1θ=k1k2c/(1+k2c)(3)
where υ is the reaction rate, c the concentration of reactant, k1 the apparent constant of reaction rate and k2 the constant of absorption equilibrium. For very low concentration of reactant, k2c1. It can be written as
υ=k1k2c(4)
ln(c0/c)=k1k2t=Kt(5)
It presents first order reaction and ln(c0/c)—t is in linear relation, where K=k1k2 is apparent first order reaction constant.
Under the conditions of 0.125% Ag/TiO2 catalyst(dry in 80℃), DMM initial concentration 100mg/L, catalyst dosage 1.25g/L, neutral condition, reaction temperature 26℃ and total volume 400mL, DMM is degraded under different lights, photocatalyst and oxidant. The experimental results are shown in Fig.6. It can be seen that the varying curve of degradation rate with time(lnc0/c—t) follows the first order kinetics reaction better.
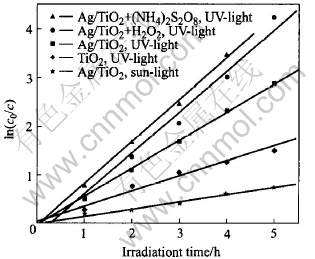
Fig.6 Plots of ln(c0/c) vs time for different reaction conditions
In the system where Ag/TiO2 used as catalyst, apparent rate constants of DMM are 0.5657h-1 and 0.1263h-1(rate constant of DMM is 0.0523h-1 without photocatalyst) respectively by UV-light and sunlight, correlation coefficients (r) are 0.9975 and 0.9984, and degradation rate constant is 4.15∶1. When 2mmol H2O2 and (NH)2S2O8 are added, reaction rate constants are respectively increased to 0.8283h-1 and 0.8350h-1, and correlation coefficient (r) are respectively 0.9881 and 0.9982. In the case that pure TiO2 is used, apparent rate constant is 0.2966h-1 (correlation coefficient 0.9944). The apparent rate constant of Ag/TiO2 catalysts is 1.9 times as much as that of pure TiO2.
3.3 Degraded pathway of DMM
The construction of DMM is characterized by a big conjugated system which is generated by two benzenes connected via α, β-unsaturated ketone. It has strong absorption at UV region (maximum absorption wavelength is 243nm). Degradation pathway is shown on Fig.7. During the procedure of degradation, double bond of ethylene out of benzene first breaks. Then unstable derivation of diphenyl occurs. Diketone is oxidized unceasingly to respectively form (1), (2) and (3). Where (1) is the rearrangement product of broken double bond. And N,O heterocycle is unstable group which can be oxidized easily to small molecule. The compound of a series of benzoic acids which are oxidized unceasingly to (4) and (6), becomes diatomic phenol (5) due to imported ·OH. Rest substituent on benzenes is oxidized ulteriorly by ·OH to become polytomic phenol (7), (8), which are oxidized easily to become biatomic acid. Biatomic acid and small molecules are mineralized entirely to become CO2 and waters at last.
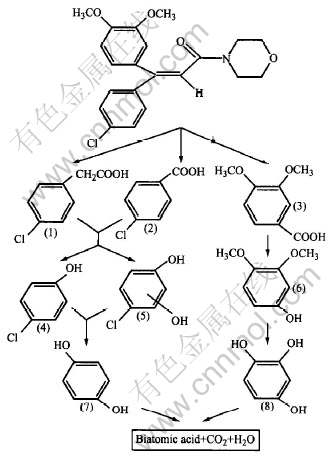
Fig.7 Possible degradation pathway of DMM
Chromatographic peak of GC-MS indicates that, after 1h reaction, the peak of DMM (tR values of Z, E isomer are 5.642 and 6.545 respectively) obviously decreases. Two small peaks appear at tR=2.393 and 3.116 and relative molecular masses are 156 and 181, namely compound (2) and (3) respectively. After 3h, peak of DMM goes on decreasing when the peak of compound (2) and (3) is increased, at the same time, peak of compound (1) (tR=2.842 and relative formula mass 170) appears. After 5h, peak of DMM basically disappears and those of (1), (2), (3) are fallen down markedly, At the same time there are a series of superposed peaks appeared between tR=1.5 and tR=2.4. The superposed peaks correspond to ramification of phenol.
4 CONCLUSIONS
1) It is found that degradation rate of DMM is 94% after UV-light irradiating for 5h under the experimental conditions. Degradation reaction follows kinetics of the apparent first order reaction. Degradation rate constant is 4.15 times for sunlight, and 1.9 times for pure TiO2 in UV-light.
2) Under UV-light condition, the optimum dosage of catalysts is 1.25g/L and the effect of degradation will be better with acidic condition. The smaller the total volume, the larger the reaction rate. And peroxide will greatly improve the reaction rate.
3) For degradation of DMM, the double bond of ethylene out of benzeon first breaks to form benzene carbonic acid, and decarboxylase into phenol, at last, mineralize benzenes into CO2 and water.
REFERENCES
[1]Tìmea P, Imre D. Photocatalytic degradation of hydrocarbons by bentonite and TiO2 in aqueous suspensions containing surfactants[J]. Colloids and Surfaces A: Physicochem Eng Aspects, 2004, 230: 191-199.
[2]Topalov A S, ojic D V, Molnár-Gábor D A. Photocatalytic activity of synthesized nanosized TiO2 towards the degradation of herbicide mecoprop[J]. Applied Catalysis B: Environmental, 2004, 54: 125-133.
[3]Bauer C, Jacques P, Kalt A. Photooxidation of an azo dye induced by visible light incident on the surface of TiO2[J]. J Photochem Photobiol A, 2001, 140: 87-92.
[4]Huang Q, Hong Chiaq-Swee. TiO2 photocatalytic degradation of PCBs in soil-water systems containing fluoro surfactant[J]. Chemosphere, 2000, 41: 871-879.
[5]Malato S, Blanco J, Vidal A, et al. Photocatalysis with solar energy at a pilot-plant scale: an overview[J]. Appl Catal B, 2002, 37: 1-15.
[6]Muruganandham M, Swaminathan M. Solar photocatalytic degradation of a reactive azo dye in TiO2-suspension[J]. Solar Energy Materials & Solar Cells, 2004, 81: 439-457.
[7]Vulliet E, Emmelin C, Chovelon J-M, et al. Photocatalytic degradation of sulfonylurea herbicides in aqueous TiO2[J]. Appl Catal B, 2002, 38: 127-137.
[8]Dumitriu D, Bally A R, Ballif C, et al. Photocatalytic degradation of phenol by TiO2 thin films prepared by sputtering[J]. Appl Catal B, 2000, 25: 83-92.
[9]Modestov A D, Lev O. Photocatalytic oxidation of 2,4-dichlorophenoxyacetic acid with titania photocatalyst—Comparison of supported and suspended TiO2[J]. J Photochem Photobiol A, 1998, 112: 261-270.
[10]HUANG Qing-chun, YE Zhong-yin. Character and mode of action of dimethomorph[J]. Pesticide Science and Administration, 2000, 21: 28-31. (in Chinese)
[11]YAN Jian-hui, HUANG Ke-long, SI Shi-hui, et al. Preparation of photocatalytic activity nanosized TiO2 by hydrolysis-sol Method[J]. The Chinese Journal of Nonferrous Metals, 2003, 13(3): 788-792. (in Chinese)
[12]ZHANG Li-de, MOU Ji-mei. Nano-Material and Nano-Structure[M]. Beijing: Science Press, 2002, 85. (in Chinese)
[13]MA Wan-hong, CAI Ru-xiu, LIU Zhi-hong, et al. UV-Vis adsorption spectra tracing on photodegradation of azo compounds on the surface of titanium dioxide—The discovery and kinetic behaviors researching of intermediates[J]. Chemical Journal of Chinese Universities, 1999, 20(10): 1542-1547. (in Chinese)
[14]Ku Y, Leu R, Lee K-C. Decomposition of 2-chlorophenol in aqueous solution by UV irradiation with the presence of titanium dioxide[J]. Water Res, 1996, 30, 2569-2578.
[15]Garcia J C, Takashima K. Photocatalytic degradation of imazaquin in an aqueous suspension of titanium dioxide[J]. J Photochem Photobiol A: Chem, 2003, 155: 215-222.
[16]Saquib M, Muneer M. TiO2-mediated photocatalytic degradation of a triphenylmethane dye (gentian violet), in aqueous suspensions[J]. Dyes and Pigments, 2003, 56, 37-49.
[17]Wang K H, Hsieh Y H, Chou M Y, et al. Photocatalytic degradation of 2-chloro and 2-nitrophenol by titanium dioxide suspensions in aqueous solution Chang[J]. Appl Catal B, 1999, 21: 1-8.
[18]Bhatkhande D S, Pangarkar V G, Beenackers A C. Photocatalytic degradation of nitrobenzene using titanium dioxide and concentrated solar radiation: chemical effects and scale up[J]. Water Research, 2003, 37: 1223-1230 .
(Edited by YANG Bing)
Foundation item: Project(2001AA2180041) supported by Hi-tech Research and Development Program of China
Received date: 2004-09-30; Accepted date: 2004-12-30
Correspondence: HUANG Ke-long, Professor, PhD; Tel: +86-731-8879850; E-mail: huangkelong@yahoo.com.cn


