J. Cent. South Univ. (2012) 19: 855-862
DOI: 10.1007/s11771-012-1083-5![]()
Microstructures and thermal properties of
municipal solid waste incineration fly ash
LIU Yuan-yuan(刘元元)1, WANG Jia-jia(王佳佳)1, LIN Xiang(林祥)2,
WANG Li-ao(王里奥)2, ZHONG Shan(钟山)3, YANG Wei(杨威)2
1. Key Laboratory of The Three Gorges Reservoir Region’s Eco-environmental of Ministry of Education,Chongqing University, Chongqing 400044, China;
2. College of Resources and Environmental Science, Chongqing University, Chongqing 400044, China;
3. College of Environment and Resources, Guangxi Normal University, Guilin 541004, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract:
To analyze the feasibility of utilization of thermal technology in fly ash treatment, thermal properties and microstructures of municipal solid waste incineration (MSWI) fly ash were studied by measuring the chemical element composition, specific surface area, pore sizes, functional groups, TEM image, mineralogy and DSC-TG curves of raw and sintered fly ash specimens. The results show that MSWI fly ash particles mostly have irregular shapes and non-typical pore structure, and the supersonic treatment improves the pore structure; MSWI fly ash consists of such crystals as SiO2, CaSO4 and silica-aluminates, and some soluble salts like KCl and NaCl. During the sintering process, mineralogy changes largely and novel solid solutions are produced gradually with the rise of temperature. Therefore, the utilization of a proper thermal technology not only destructs those persistent organic toxicants but also stabilizes hazardous heavy metals in MSWI fly ash.
Key words:
municipal solid waste incineration; fly ash; thermal treatment; sintering; microstructure;
1 Introduction
Incineration has been widely recognized as an efficient method for municipal solid wastes (MSW) treatment. It is able to lead to an 80% volume reduction and a 60%-75% mass decrease, and can destroy pathogenic agents and recover energy [1-2]. However, the treatment of MSWI fly ash somehow is a critical factor to the application of incineration to deal with municipal solid waste problem. MSWI fly ash, one of the main secondary pollutants from municipal solid waste incineration process, is seen as a kind of hazardous wastes in many countries owing to its high concentration of leachable toxic heavy metals and the presence of chlorinated organic compounds [1].
MSWI fly ash is a complex mixture of various minerals [3]. Small pellets associated with aggregates of polycrystalline, amorphous and glassy material are commonly found in MSWI fly ash [4-5]. They usually consist of complex calcium, sodium and potassium aluminosilicates while the associated amorphous and crystalline material are enriched in more volatile heavy metals [6]. MSWI fly ash is regulated to be pretreated before final disposal in many countries.
Chemical stabilization, acidic solution, solidification and thermal treatment are already employed to pretreat MSWI fly ash [7]. Chemical stabilization transforms mobile heavy metals into hardly dissolvable and low-toxic forms through chemical reactions with sodium hydroxide, sodium sulfide, EDTA and some other chemical stabilizers [7-8]. Acidic extraction is actually a hydrometallurgical process as most of the metals may be dissolved in an acidic solution and then separated. However, KASTUURA et al [9] determined that acidic extraction leachates are very difficult to filter out because of the formation of colloidal silicates and aluminates. Solidification technology involves trapping hazardous waste using inert materials through physical and chemical methods and thus stabilizing them [10]. However, due to the complexity of the heavy metals and other compositions of MSWI fly ash, it is very difficult to find a cost effective stabilization remediation method [11]. In such a complex system, the microstructure of MSWI fly ash will, to a great extent, influence the leachability of pollutants such as heavy metals.
Thermal treatment may reduce the volume of fly ash and meanwhile can destroy the hazardous organic components while being highly effective in heavy metal stabilization [12]. Recently, the sintering technology attracts more attention, because of lower expense than vitrification due to its lower heating temperature and the complete destruction of organic pollutants like PCDD/Fs [12]. However, some of the heavy metals such as Cd and Pb compounds and inorganic salts in the fly ash during thermal treatment may be volatilized when being heated due to their low melting points [13].
In this work, the microstructure of MSWI fly ash and thermal properties when fly ash is heated are studied to explore the influential factors and feasibility of a treatment method by means of observing the TEM image, mineralogy and DSC-TG curves of MSWI fly ash.
2 Experimental
2.1 Materials
MSWI fly ash samples were collected from the gas dust filter of a MSWI plant in Chongqing. The flue gas clean-up system includes a dust collection cyclone, a scrubber and a baghouse filter. Large particles in MSWI flue gas would normally be removed by dust collection cyclone; in scrubber, certain amounts of lime slurry and activated carbon are introduced to remove acidic gas and gaseous organic pollutants. The mixed MSWI fly ash samples were collected at baghouse outlet. The MSWI fly ash was ground to fine particles (<200 μm) to ensure the homogeneity for the measurements and observations.
2.2 Experimental methods
Raw sample of MSWI fly ash was observed with transmission electron microscope (TEM) to obtain its micro appearance. Specific surface area, pore sizes and distribution of raw and sonicated samples were measured by physical absorbance instruments through measuring and calculating N2 gas static adsorption capacity based on BET equation and BJH methods.
Fly ash samples were oven-heated at 850, 900, 1 000 and 1 050 °C in a muffle furnace for 1 h. The mineralogy of raw and heated fly ash was measured by X-ray powder diffraction (XRD). Differential scanning calorimetry (DSC) and thermo-gravimetric analysis (TG) curves were measured by thermal analyzer in air by heating the samples from 25 °C to 1 300 °C at the rate of 20 °C/min. Before these analyses, the MSWI fly ash samples were ground to less than 150 μm.
2.3 Analysis
The elemental composition of RFA was determined by Finder 1000 X-ray energy spectrometer (XRF). TEM analysis was conducted by the Philips TECNAI 10 TEM microscope (100 kV). The particle sizes of MSWI fly ash were measured by a Microtrac FLEX 3500 laser particle size analyzer. Specific surface area, pore sizes and distribution measurement of raw and sonicated samples were conducted by physical absorbance instruments (ASAP 2020 M, Micrometrics Co.) by measuring N2 gas static adsorption capacity and calculated based on BET equation and BJH methods.
DSC-TG analysis was conducted on fly ash in an Al2O3 crucile with a NETZSCH STA 449 C thermal analyzer, using powder alumina as the reference material. The media atomerspher is air and flow rate is 30 mL/min, and argon was used as protection medium with a flow of 10 mL/min.
The crystalline phases presented in the samples were characterized using Cu Kα radiation (40 kV, 100 mA). XRD analysis was performed using a BDX3200 diffractometer with Cu Kα (λ=1.540 56 ?) radiation. All samples were adhered into a zero background sample holder with scanning 2θ angles from 3° to 80° using a step interval of 0.02° and a scan speed of 2 (°)/min. The XRD patterns were processed using the computer program MDI Jade 6.5 (Materials Data, Inc., Livermore, CA).
3 Results and discussion
3.1 Chemical composition
According to the previous studies [14-15], the main elements in MSWI fly ash are Si, Ca, K, Na, Al, Cl, Fe, Ti and Mg, and the main toxic heavy metals include Pb, Cr, Cd, Zn, Hg, Cu, Ni and As. The elemental compositions of the MSWI fly ash samples used in this work are summarized in Table 1. It is notable that O, Si and Ca, accounting for 30%, 22% and 12%, respectively, are the major elements. The high concentration of Ca in this fly ash is attributed to the Ca(OH)2 solution sprayed in the acid scrubber. Si, Cl, C, Na, S, K, Al, Fe, Mg and P are the next most abundant elements, each comprising about 1%-8%.
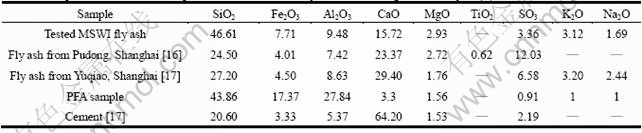
The chemical compositions of the MSWI fly ash are compared to other MSWI fly ash samples [16-17], PFA sample and cement in Table 2.
The main compositions belong to CaO-SiO2-Al2O3- Fe2O3 systems. As shown in Table 2, MSWI fly ash and PFA have similar compositions. The content of SiO2 is as high as 46.6%, higher than that of the fly ash samples from other regions in China. The contents of Al2O3 and Fe2O3 are close to fly ash samples from other regions, while the content of CaO of 16% is much higher than PFA, and lower than MSWI samples from other regions.
3.2 Particle size and distribution
The sizes of MSWI fly ash determine the application of the technologies for the treatment and beneficial utilization of MSW fly ash. First, MSWI fly ash was separated by 100 mesh screen, and then the particles less than 150 μm in MSWI fly ash samples were measured by laser particle size analyzer. The fly ash with >150 μm particles represents 10.67%, while the particles <150 μm occupies 89.33%.
Table 1 Elemental compositions of MSWI fly ash (mass fraction, 10-6)
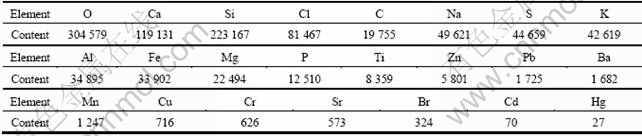
Table 2 Comparison of chemical composition between MSWI fly ashes and PFA (pulverized fly ash) (mass fraction, %)
As shown in Fig. 1, the particle sizes of MSWI fly ash (<150 μm) represent a normal distribution. The sizes of most particles in MSWI fly ash are less than 250 μm with an average of 75.18 μm; the particles less than 248.9 μm occupy 99.63%. The sizes of most particles are in the range of 25-250 μm, and the sizes of particles in the range 26.2-248.9 μm represent 79.2%. Therefore, MSWI fly ash can be utilized as fine particle materials.
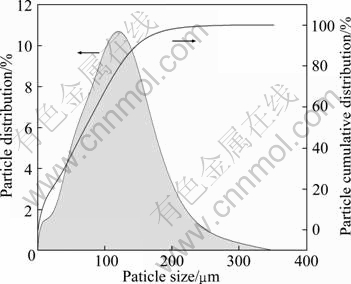
Fig. 1 Particle size distribution of MSWI fly ash (under 100 mesh screen)
3.3 Microstructures
3.3.1 Specific surface areas and pore structure
The specific surface area represents the reactive interface and active sites of a matter, and pore structure shows the mass transfer channel. The two parameters are of importance similar to chemical compositions of a matter [18]. The BET specific area and BJH pore sizes of tested MSWI fly ash are given in Table 3. Raw MSWI fly ash samples have a small specific surface area of 1.931 4 m2/g. The pore size is 19.606 8 nm averagely; pore volume is 0.008 421 cm3/g. The structure of the MSWI fly ash particle is not a typical porous structure and the maximum absorption capacity is 5.444 2 cm3/g STP at relative pressure of 0.994 0 (Fig. 2). After being sonicated in DI water for 30 min, the specific surface area and pore volume of MSWI fly ash sample are increased almost 10 times to 18.036 8 m2/g and 0.080 518 cm3/g, respectively, because the fine matters absorbed physically on the surface or in the pores of MSWI fly ash aggregates are separated from the matrix or the blockage in the pores in MSWI fly ash aggregates is removed through the cavitation effect of sonication.
Table 3 Specific surface areas (BET), pore size and pore volume of MSWI fly ash

As shown in Fig. 2, the desorption/absorption curves of raw MSWI fly ash represent “S” shape and belong to isotherm of type II absorption, which denotes that the absorptions are free single multilayer reversible absorption on non-porous solid surface or solids with large pores. The desorption/absorption curves of sonicated MSWI fly ash have an apparent hysteresis loop, which means that the sonicated MSWI fly ash samples have typical porous structure. The maximum absorption capacity is increased up to 52.054 7 cm3/g STP at the relative pressure of 0.994 2, which denotes that the absorption capacity of MSWI can be enhanced by sonication. On the curve of the desorption/absorption curves of sonicated MSWI fly ash, the absorption curve increases suddenly and the desorption curve changes slowly in the mediate range of relative pressure, which is the typical hysteresis loop of a structure with heterogeneously distributed pores. Therefore, the pore structure of MSWI fly ash is mostly taper hole or dual taper capillary shape hole or uniform hole with open ends and closed sides.
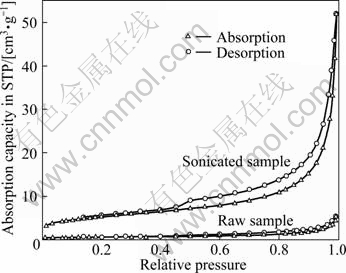
Fig. 2 Isotherm desorption/absorption curves of raw and supersonic MSWI fly ash
3.3.2 TEM microscopy
The MSWI fly ash samples were ground to less than 45 μm and dispersed to a suspension by alcohol as dispersant. A few droplets of suspension were dropped on a copper screen. After being air-dried, the MSWI fly ash on the copper screen was observed by TEM.As shown in Fig. 3, MSWI fly ash samples are mainly composed of irregular shape particles. Figure 3(a) shows a large number of nano-wire and some plate shape particles. The analysis based on the contrast grade of the image shows the porous structure of the irregular shape particles of MSWI fly ash. There are a small number of plate shape particles which usually are the typical structure of clay minerals. Figure 3(b) shows that round-plate particles are surrounded by colloid matter. MSWI fly ash particles as a whole represent coral reef shape, which perhaps are the aggregated particles from small ones.
As a comparison, the MSWI fly ash ground to less than 45 μm was sonicated in DI water for 30 min, and the solids air-dried were then observed through TEM, as shown in Fig. 4.
The TEM images of sonicated MSWI fly are shown in Fig 4. As shown in Fig. 4(a), the particles with irregular shape are dispersed well. Most particles look black and gray, and have a high contrast grade, which indicates the non-porous structure. However, Fig. 4(b) shows more regular structure improved by sonication. Particles have clear boundaries, regular shape and relatively even thickness. Some round zones in dark attached on some thin plates have relatively small contrast grades compared to the surrounding areas. They could be caused by the oxides of heavy metals with a large atomic number, and the leaching of these heavy metals imposes many kinds of hazards. Through immobilizing these matters in a certain structure, the leaching out from MSWI fly ash could be effectively blocked.
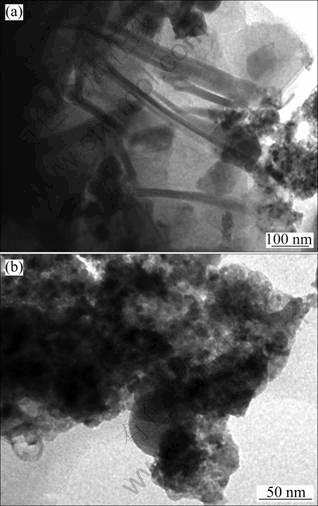
Fig. 3 TEM images of raw MSWI fly ash
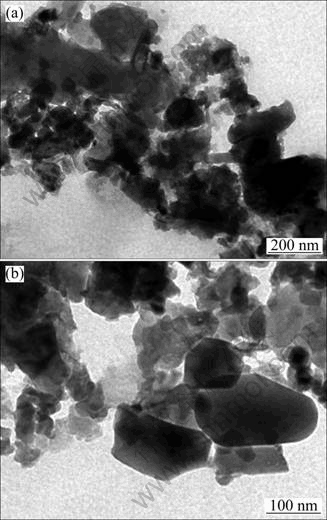
Fig. 4 TEM images of sonicated MSWI fly ash
3.4 Thermal properties
MSWI fly ash is composed of many kinds of silicates and aluminates, which are commonly used as raw materials during ceramics production and can form the skeleton structure of the ceramic materials. The vitric materials and active metal ions activating network make MSWI fly ash have a certain reactive activity. The sizes of particles are small enough to save a large amount of energy to achieve mass and heat transfer for beneficial utilization of MSWI fly ash. The thermal properties of MSWI fly ash were studied through observing the change of parameters such as mass loss, crystal phases and mineral compositions during heating from room temperature to 1 300 °C.
3.4.1 DSC-TG curves
The measurements of DSC-TG curves were applied to analyzing the physical processes including crystal lattice transformation, sublimation, and chemical processes including melting and reduction, oxidation and decomposition when MSWI fly ash is heated at various temperatures. The synchronous measurement of differential scanning calorimetry and thermo- gravimetric analysis can used to analyze the endo/exothermic processes, and meanwhile can record the mass loss at the corresponding heating temperatures. The DSC-TG synchronous curves of MSWI fly ash from 25 to 1 300 °C are shown in Fig. 5.
It can be seen from the TG curve in Fig. 5 that two findings feature the heating process of MSWI fly ash: 1) The mass loss rate is high up to 15.10% when MSWI fly ash is heated from room temperature (25 °C) to 1 300 °C; 2) The TG curve is composed of three single-step processes and four platforms, where each single step process represents a mass loss while the platform responds to a certain stable compound. The range of 33.0-188.0 °C is the first single-step process with an apparent mass loss of 2.12%; the range of 188.0-715.0 °C is the second single-step process with mass loss of 3.08%; the range of 715.0-1 293.0 °C is the third single-step process, which contributes to the main mass loss of MSWI fly ash during heating, 9.90%. Therefore, it is speculated the main chemical process takes place in the third single-step process.
The DTG curve in Fig. 5 can be obtained by calculating the first order derivative of TG curve, which represents the rate of mass change. The peaks on the DTG curve means the maximum values of mass change rate responding to the flex points on TG curve. For a reaction taking place during the heating process, the starting temperatures, the temperature of the maximum rate and terminal temperatures can be easily identified from DTG curve, and the height of a peak is believed to be the reaction rate at that temperature. From the DTG curve, it is found that three maximum reaction rates of MSWI fly ash take place at the temperatures of 64.2, 683.8 and 1 165. 3 °C.
The DSC curve shows five peaks including one exothermic and four endothermic processes in the whole heating range from 25 to 1 300 °C. The temperature range of 350-500 °C with the exothermic peak at 460 °C could represent the chemical oxidation process of unburned materials in MSWI fly ash at relatively low temperatures. The first endothermic process takes place at 25-106.0 °C with a peak at 72.5 °C, which represents the removal of absorption water in MSWI fly ash, that is, the drying of MSWI fly ash; the second endothermic process take place at 581.0-626.0 °C with a weak peak at 604.1 °C, which represents the transformation process of crystals in MSWI fly ash. The third endothermic process takes place at 659.0-712.0 °C with a peak at 687.3 °C, which shows the decomposition process of compounds with a low decomposition temperature in MSWI fly ash, such as Ca(OH)2. In addition, the vitrification and volatilization of some MSWI materials belonging to endothermic processes take place in this temperature range. The fourth endothermic process on the DSC curve of MSWI fly ash starts at 1 141.4 °C and ends at 1 188.1 °C. The endothermic peak is at 1 168.7 °C. This endothermic process corresponds to the melting reaction of MSIW fly ash. This indicates that the materials with low melting points could have finished their melting processes at this temperature. The above observations indicate that the melting process is the most intensive endothermic process when MSWI fly ash is heated from 25 to 1 300 °C.
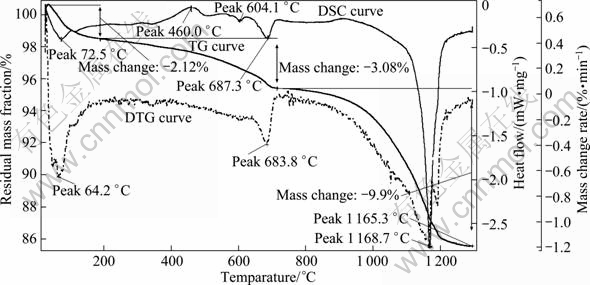
Fig. 5 DSC-TG-DTG curves of raw MSWI fly ash
The relationship between mass change and physical and chemical process can be explained through the synchronous DSC-TG curves. The mass loss of MSWI fly ash in the temperature range of 33.0-188 °C is equal to 2.12%, which can be attributed to endothermic process in the temperature range of 25-106.0 °C. Hence, this mass loss could be attributed to the removal of absorption and crystal water within MSWI fly ash. With the increase of heating temperature, another mass loss of 3.08% takes place in the temperature range of 188.0-715.0 °C, which corresponds to the exothermic process in the temperature range of 350-500 °C, and two endothermic processes in the temperature ranges of 581.0-626.0 °C and 659.0-712.0 °C. Three processes are relatively weak, and the overall processes display an endothermic effect because the heat released by the exothermic processes can offset by the two endothermic processes. The endothermic processes such as hydrate removal in the temperature range of 300-1 000 °C, the decomposition of carbonates at temperature above 600 °C, the transformation of quartz at about 570 °C, and the melting of chlorides of metals with low melting points ZnCl2 (283 °C), PbCl2 (501 °C), CdCl2 (568 °C), CuCl2 (620 °C) could be combined in this overall endothermic process.
In the whole heating process, the largest mass loss on the TG curve, 9.9%, takes place in the high temperature range of 715-1 293 °C, which corresponds to endothermic process in the temperature range of 914.0-1 288 °C. During the heating in this temperature range, plenty of heat is absorbed by MSWI fly ash, which is attributed to the processes such as the decomposition of carbonates and sulfates, and the volatilization of chlorides of heavy metals with high volatilization points such as CuCl2 (993 °C), PbCl2 (950 °C), CdCl2 (960 °C), ZnCl2 (732 °C) according to the chemical compositions of MSWI fly ash.
The coupling analysis of DSC-TG-DTG curves shows the complex physical and chemical process during the whole heating process. Generally, MSWI fly ash stays relatively stable at the temperature below 1 000 °C, and the main processes take place in the temperature range of 1 000-1 250 °C, during which some constituents are decomposed, some are volatilized and main constituents are melted. MSWI fly ash undergoes the processes of dewatering, crystal transformation, decomposition, volatilization and melting in the temperature sequence when being heated from room temperature 25 to 1 300 °C, while the melting process plays a major role.
3.4.2 Crystal phases and compositions
As shown in Fig. 6, the phase compositions of MSWI fly ash are fairly complex and the major crystal phases are SiO2, CaSO4 and some silico aluminates. In addition, some hydrated CaSO4 crystals can be found, which may be attributed to the reaction between the SO2 gas and slime slurry injected to acid scrubber at high temperatures. Some dissolvable salts such as KCl and NaCl can also be identified from the spectrum. Some acromions appear on the MSWI fly ash XRD spectrum, which indicates that the existence of amorphous constituents is perhaps caused by produced melt inorganic or unburned carbon in MSWI fly ash.
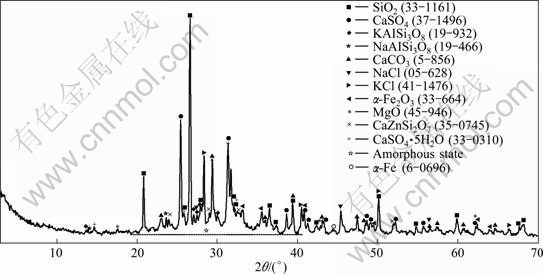
Fig. 6 XRD pattern of MSWI fly ash
The heating temperatures would influence significantly the crystal phases of MSWI fly ash [19]. The crystal phases of MSWI fly ash heated at 850, 900, 1 000 and 1 050 °C are compared with raw MSWI fly ash, as shown in Fig. 7.
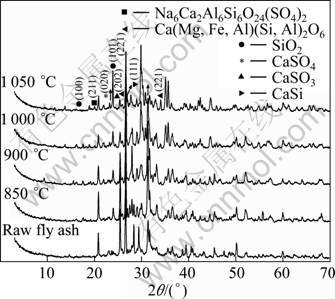
Fig. 7 XRD patterns of MSWI fly ashes isothermally sintered at various temperatures
As shown in Fig. 7, the main phase compositions change apparently after being heated at various temperatures. Heating at temperature above 850 °C removes hydrate or absorption water, combust organic and decompose decomposable matters so that some constituents such as Ca(OH)2, CaCO3, CaSO4·5H2O disappear. By comparing the intensity of diffraction peaks, it is found that the contents of а-Fe2O3, а-Fe, CaSO4, CaSi, CaSiO3, SiO2, NaAlSi3O8/KAlSi3O8 are increased after being heated at high temperatures. With the increase of heating temperatures, the intensity of diffraction peaks of also α-Fe2O3 (104) is increased first and then decreased, while that of α-Fe (110) keeps increasing, which may be attributed to the reduction effect on Fe2O3 of reductive atmosphere. In addition, the diffraction intensities of CaSO4 (020), SiO2 (101) (100), NaAlSi3O8 (040), SiO2 (101) and CaSi (111) are weakened, especially the diffraction intensity of CaSi (111) and SiO2 (101) at 1 000 °C and 1 050 °C. The diffraction intensity of Na6Ca2Al6Si6O24(SO4)2 (211) and Ca(Mg, Fe, Al) (Si, Al)2O6 (221) is increased apparently, and that of CaSiO3 (320) is increased slightly with the increase of heating temperature. Solid solutions such as Na6Ca2Al6Si6O24(SO4)2 and Ca(Mg, Fe, Al)(Si, Al)2O6 are produced through the reaction between the components in MSWI fly ash such as CaSi, CaSO4, SiO2 and feldspar (NaAlSi3O8). The diffraction intensity of CaSiO3 is increased with the increase of heating temperature, perhaps because the degree of crystallinity is increased due to the higher temperature or CaSi is oxidized to produce new CaSiO3.
The original phase compositions of raw MSWI fly ash such as CaCO3, KCl, NaCl, MgO and CaSO4·5H2O react with each other and then disappear. New crystal phases Na6Ca2Al6Si6O24(SO4)2 and Ca(Mg, Fe, Al) (Si, Al)2O6 appear and the diffraction intensity is increased with the increase of heating temperature. When MSWI fly ash is heated to 1 000 °C, the diffraction intensity of SiO2 (101) is decreased apparently, which indicates that SiO2 is involved in the reaction to produce Na6Ca2Al6Si6O24(SO4)2. Due to the complexity of the composition of MSWI fly ash, the low content compositions are hard to be identified from XRD spectra.
4 Conclusions
1) MSWI fly ash samples are mainly composed of fine irregular shape particles and do not have a typical porous structure. Raw MSWI fly ash samples have a small specific surface area, pore size and pore volume, however, the specific surface area and pore volume of MSWI fly ash sample are increased almost 10 times after being sonicated. Sonication can improve the porous structure of MSWI fly ash. The particle sizes of MSWI fly ash (<150 μm) represent a normal distribution. The sizes of most particles in MSWI fly ash are less than 250 μm with an average of 75.18 μm; while the particles less than 248.9 μm occupy 99.63%.
2) The mass loss is high up to 15.10% when MSWI fly ash is heated from room temperature (25 °C) to 1 300 °C, and three maximum reaction rates of MSWI fly ash take place at the temperatures of 64.2, 683.8 and 1165. 3 °C. MSWI fly ash undergoes the processes of dewatering, crystal transformation, decomposition, volatilization and melting in the temperature sequence when being heated from room temperature (25 °C) to 1 300 °C, while melting process plays a major role.
3) The original phase compositions such as CaCO3, KCl, NaCl, MgO and CaSO4·5H2O disappear, while new crystal phases Na6Ca2Al6Si6O24(SO4)2, Ca(Mg, Fe, Al) (Si, Al)2O6 and Mg3Si4O10OH2 are generated and the diffraction intensity is increased with the increase of heating temperatures. Solid solutions with a large skeleton structure such as Na6Ca2Al6Si6O24(SO4)2 and Ca(Mg, Fe, Al) (Si, Al)2O6 can be produced from the reactions involving oxides of silica, alumina and other metals in MSWI fly ash when MSWI fly ash is heated at high temperatures.
References
[1] WAN Xiao, WANG Wei, YE Tun-min. A study on the chemical and mineralogical characterization of MSWI fly ash using a sequential extraction procedure [J]. Journal of Hazardous Materials, 2006, 134(1/2/3): 197-201.
[2] LIU Yang-sheng, ZHENG Li-ting, LI Xiao-dong. SEM/EDS and XRD characterization of raw and washed MSWI fly ash sintered at different temperatures [J]. Journal of Hazardous Materials, 2009, 162(1): 161-173.
[3] ZHAO Guang-jie, LI Hai-bin, ZHAO Zeng-li, YAN Chang-feng, CHEN Yong. Basic properties of fly ash from incineration of municipal solid waste [J]. Journal of Fuel Chemistry and Technology, 2005, 33(2): 184-188. (in Chinese)
[4] KIRBY C S, RLMSTLDT J D. Mineralogy and surface properties of municipal solid waste ash [J]. Environ Sci Technol, 1993, 27(4): 652-660.
[5] ONTEVAROS J L, CLAPP T L, KOSSON D S. Physical properties and chemical species distributions within municipal waste combustor ashes [J]. Environ Progress, 1989, 8(3): 200-206.
[6] EIGHMY T T, EUSDEN J D, KRZANOWSKI J E, DOMINGO D S, STAMPFLI D, MARTIN J R, ERICKSON P M. Comprehensive approach toward understanding element speciation and leaching behavior in municipal solid waste incineration electrostatic precipitator ash [J]. Environ Sci Technol, 1995, 29(3): 629-646.
[7] ZHANG Da-jie, LIU Wen-shi, HOU Hao-bo. Strength, leachability and microstructure characterisation of Na2SiO3-activated ground granulated blast-furnace slag solidified MSWI fly ash [J]. Waste Management & Research, 2008, 25(5): 402-407.
[8] ZHAO You-cai, SONG Li-jie, LI Guo-jian. Chemical stabilization of MSW incinerator fly ashes [J]. Journal of Hazardous Materials B, 2002, 95: 47-63.
[9] KASTUURA H, INOUE T, HIRAOKA M, SAKAI S. Full-scale plant study on fly ash treatment by the acid extraction process [J]. Waste Management, 1996, 16: 491-499.
[10] KARAMANOV A, PELINO M, HREGLICH A. Sintered glass- ceramics from municipal solid waste-incinerator fly ashes-part I: The influence of the heating rate on the sinter-crystallisation [J]. J Eur Ceram Soc, 2003, 23(6): 827-832.
[11] YAN Jian-hua, MA Zeng-yi, PENG Wen, LI Xiao-dong, LI Jian-xin, CEN Ke-fa. Experimental study on solidification of MSW incinerator fly ash by mixing with asphalt [J]. Acta Scientiae Circumstantiae, 2004, 24(4): 71-74. (in Chinese)
[12] JIN Chong-yang, CUI Di-chen. Discussion on solid wastes incineration residues treatment technology [J]. Science of Environment Protection, 2003, 4: 32-35. (in Chinese)
[13] ZHANG Hai-ying, ZHAO You-cai, QI Jing-yu. Study on use of MSWI fly ash in ceramic tile [J]. Journal of Hazardous Materials, 2007, 141(1): 106-114.
[14] TAKAOKA M, TAKEDA N, MIARA S. The behaviour of heavy metals and phosphorus in an ash melting process [J]. Water Science and Technology, 1997, 36: 275-282.
[15] ZHANG Hai-ying. Utilization of MSWI fly ash in the production of ceramic tile [D]. Shanghai: Tongji University, 2005: 34-37. (in Chinese)
[16] HE Pin-jing, ZHANG Hua, CAO Qun-ke, ZHANG Pei-jun. Characterization of APC residues from Shanghai Pudong waste-to- energy facility [J]. Environmental Chemistry, 2004, 23(1): 38-42. (in Chinese)
[17] YUAN Ling, SHI Hui-sheng, YUE Peng. Research on potential cementitious reactivity of fly ash from incinerator of municipal solid wastes [J]. Journal of Tongji University: Natural Science, 2003, 12(31): 1444-1448. (in Chinese)
[18] YUE Peng, SHI Hui-sheng, SHU Xin-ling. Preliminary research on cementitious activities of municipal solid wastes incineration ash [J]. Cement, 2003(5): 12-15. (in Chinese)
[19] KARAMANOV A, PELINO M, HREGLICH A. Sintered glass-ceramics from municipal solid waste-incinerator fly ashes— Part I: The influence of the heating rate on the sinter-crystallisation [J]. J Eur Ceram Soc, 2003, 23(6): 827-832.
(Edited by YANG Bing)
Foundation item: Project(50808184) supported by the National Natural Science Foundation of China
Received date: 2011-07-26; Accepted date: 2011-11-14
Corresponding author: LIU Yuan-yuan, Associate Professor, PhD; Tel: +86-13883315366; E-mail: Liuyuanyuan@cqu.edu.cn
Abstract: To analyze the feasibility of utilization of thermal technology in fly ash treatment, thermal properties and microstructures of municipal solid waste incineration (MSWI) fly ash were studied by measuring the chemical element composition, specific surface area, pore sizes, functional groups, TEM image, mineralogy and DSC-TG curves of raw and sintered fly ash specimens. The results show that MSWI fly ash particles mostly have irregular shapes and non-typical pore structure, and the supersonic treatment improves the pore structure; MSWI fly ash consists of such crystals as SiO2, CaSO4 and silica-aluminates, and some soluble salts like KCl and NaCl. During the sintering process, mineralogy changes largely and novel solid solutions are produced gradually with the rise of temperature. Therefore, the utilization of a proper thermal technology not only destructs those persistent organic toxicants but also stabilizes hazardous heavy metals in MSWI fly ash.


