DOI:10.19476/j.ysxb.1004.0609.2018.03.11
氧化钇包覆LiNi0.5Co0.2Mn0.3O2的结构和电化学性能
许军娜1, 2,陈晓青2,高 雄3,周友元3,肖可颂1,黄承焕3,习小明4
(1. 金瑞新材料科技股份有限公司,长沙410012;
2. 中南大学 化学化工学院,长沙410083;
3. 湖南长远锂科有限公司,长沙410205;
4. 长沙矿冶研究院有限责任公司,长沙410012)
摘 要:
以氧化钇溶胶为包覆前驱物,利用氧化钇和正极材料表面带电状态不同制备氧化钇包覆LiNi0.5Co0.2Mn0.3O2。采用X射线衍射仪(XRD)、扫描电镜(SEM)、透射电镜(TEM)及电化学测试等手段对LiNi0.5Co0.2Mn0.3O2包覆前后的物相结构、表面形貌及电化学性能进行研究。结果表明:氧化钇包覆并未影响LiNi0.5Co0.2Mn0.3O2的晶体结构,氧化钇以颗粒状分布在正极材料表面,氧化钇包覆层厚度在15~25 nm,氧化钇在正极材料表面分布比较均匀。与未包覆LiNi0.5Co0.2Mn0.3O2相比,氧化钇包覆后,材料在高电压下的循环稳定性有所提高,最佳包覆量为0.4%。氧化钇包覆有效降低材料在充放电过程中的极化和电荷转移电阻。
关键词:
氧化钇;表面包覆;LiNi0.5Co0.2Mn0.3O2;电化学性能;
文章编号:1004-0609(2018)-03-0528-08 中图分类号:TM912.9 文献标志码:A
镍钴锰系三元材料(LiNi1-x-yCoxMnyO2)具有较高的比容量、较好的安全性能和较低的成本等,是目前正极材料研究的重点。目前,主要的三元正极材料有LiNi1/3Co1/3Mn1/3O2、LiNi0.5Co0.2Mn0.3O2、LiNi0.6Co0.2- Mn0.2O2、LiNi0.8Co0.1Mn0.1O2等,与其他三元材料相比,研究发现LiNi0.5Co0.2Mn0.3O2具有较好的稳定性和较高的比容量[1]。但是,在充放电过程中,由于锂离子脱嵌及材料与电解液的副反应导致LiNi0.5Co0.2Mn0.3O2的结构和循环稳定性降低[2-3]。特别是在高电压条件下,该现象尤为突出,限制了材料向高能量密度方向发展。表面包覆被认为是改善镍钴锰系三元材料循环稳定性的一种有效方法[4-8],其中氧化物包覆由于价格便宜、结构稳定等特点被广泛应用于正极材料包覆中[9-15]。氧化钇由于具有较好的热稳定性、较好的耐氧化和还原特性等被应用于正极材料包覆[16]。JU等[17]采用沉淀法,以NH4Cl/NH3·H2O缓冲液控制pH=9,将LiMn2O4加入到Y(NO3)3·6H2O 的缓冲溶液中,通过热处理制备了氧化钇包覆的LiMn2O4,提高了常温(25 ℃)和高温下(55 ℃)的循环性能。WU等[18]采用溶液浸渍法,将LiCo1/3Ni1/3Mn1/3O2与Y(NO3)3·6H2O的水溶液混合,干燥后于650 ℃热处理制备氧化钇包覆的LiCo1/3Ni1/3Mn1/3O2,提高了LiCo1/3Ni1/3Mn1/3O2在高电压下(4.5和4.6 V)的容量保持率。沉淀法制备过程比较复杂,溶液浸渍法相对比较简单,但是以Y(NO3)3·6H2O为钇源,热处理过程中,容易产生有害的氮氧化物。因此,急需寻找一种简单、环保的氧化钇包覆工艺。本文作者首次以市售氧化钇溶胶为包覆液,利用氧化钇溶胶和正极材料表面所带电荷不同,制备氧化钇包覆LiNi0.5Co0.2Mn0.3O2,且制备过程简单、便于规模化,并系统研究氧化钇包覆量对LiNi0.5Co0.2Mn0.3O2的晶体结构、容量和循环稳定性的影响。
1 实验
1.1 样品制备
以市售的LiNi0.5Co0.2Mn0.3O2(振实密度为2.20 g/cm3)为正极材料。将LiNi0.5Co0.2Mn0.3O2加入定量的去离子水中,然后按照包覆质量比0.2%、0.4%、0.6%加入定量氧化钇溶胶,以500 r/min速度搅拌30 min,然后将悬浊液过滤,于80 ℃真空干燥箱中烘干,置于马弗炉中于500 ℃热处理6 h得到氧化钇包覆样品。
1.2 样品表征
采用日本Rigaku公司的X射线衍射仪对样品进行物相分析,扫描范围为10°~70°,步宽为0.02°。采用美国Perknelmer Optima 2000DV 型电感耦合等离子发射光谱仪对钇含量进行测定。采用KYKY2800型扫描电镜对样品进行微观形貌分析。Tecnai G2 20型透射电子显微镜对包覆层厚度进行观察,并采用马尔文的ZS90粒度电位仪对材料的电位进行分析。
1.3 电化学性能
以LiNi0.5Co0.2Mn0.3O2为正极材料,乙炔黑为导电剂,聚偏氟乙烯(PVDF)为粘结剂,将三者按质量比90:5:5比例混合,加入溶剂N-甲基吡咯烷酮(NMP),搅拌成膏状,均匀涂覆于集流体铝箔上,于90℃干燥制成工作电极。以金属锂片为负极,Cel-gard2400微孔聚丙烯膜为隔膜,1 mol/L的六氟磷酸锂(LiPF6/EC、DMC和EMC的体积比为1:1:1)为电解液,在氩气手套箱内组装成扣式电池。以蓝电电池测试系统进行充放电测试。测试电压为3.0~4.4 V和3.0~4.6 V。以普林斯顿电化学工作站进行电化学阻抗测试,测试频率范围5 mHz~100 kHz,扰动电压为5 mV。
2 结果与讨论
2.1 XRD表征
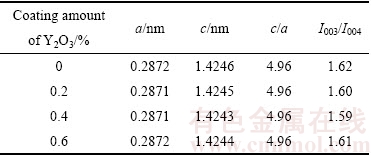
图1所示为氧化钇包覆LiNi0.5Co0.2Mn0.3O2的XRD谱。由图1中可以看出,包覆前后,衍射峰位置并未发生明显变化,仍为α-NaFeO2结构,属于六方晶系,R3m空间群,表明包覆并未影响材料的层状结构。该结构中,Li+占3a的位置,Ni2+和Mn4+占3b位置,O2-占6c位置。由于Li+半径(0.069 nm)与Ni2+半径(0.076 nm)接近,因此,Li+容易占据Ni2+位,形成阳离子混排。通常采用I003/I104衍射峰的强度(R)比来衡量阳离子混排程度。一般认为R>1.2,表明材料具有理想的层状结构和较好的电化学性能[19]。表1所列为包覆前后LiNi0.5Co0.2Mn0.3O2的晶胞常数。由表1中可以看出,包覆前后LiNi0.5Co0.2Mn0.3O2的晶胞参数及I003/I004的比值几乎没有变化,表明包覆对材料结构并未产生影响,而I003/I004均大于1.2,表明样品均具有较低的阳离子混排。
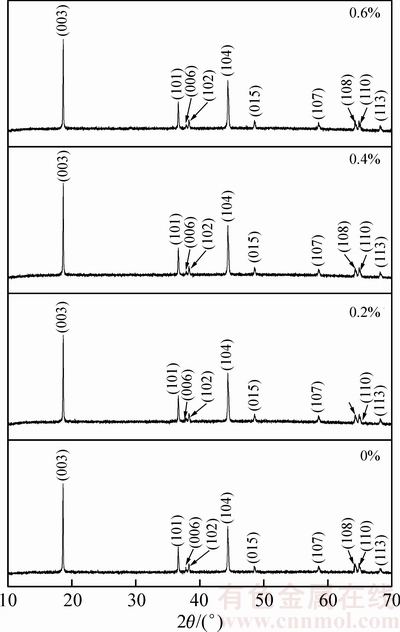
图1 氧化钇包覆LiNi0.5Co0.2Mn0.3O2的XRD谱
Fig. 1 XRD patterns of yttrium oxide coated LiNi0.5Co0.2Mn0.3O2
表1 氧化钇包覆LiNi0.5Co0.2Mn0.3O2晶胞参数
Table 1 Lattice parameter of yttrium oxide coated LiNi0.5Co0.2Mn0.3O2
2.2 形貌分析
通过电位测试仪测得氧化钇溶胶和正极材料的表面电位分别为37.5和-22.5 mV,表明氧化钇溶胶和正极材料带相反电荷,可以依靠正负电荷的吸引使氧化钇作用于正材材料表面。采用ICP-AES测得理论包覆量为0.2%、0.4%和0.6%的氧化钇包覆LiNi0.5Co0.2Mn0.3O2样品的实际包覆量为0.19%、 0.38%和0.57%,接近于理论计算值。图2所示为氧化钇包覆LiNi0.5Co0.2Mn0.3O2的SEM像。由图2中可以看出,未包覆LiNi0.5Co0.2Mn0.3O2颗粒表面比较光滑,而经氧化钇包覆后,可以看到有少量的颗粒分布在LiNi0.5Co0.2Mn0.3O2颗粒表面,且随着包覆量的增加,正极材料表面氧化钇颗粒的数量明显增加。
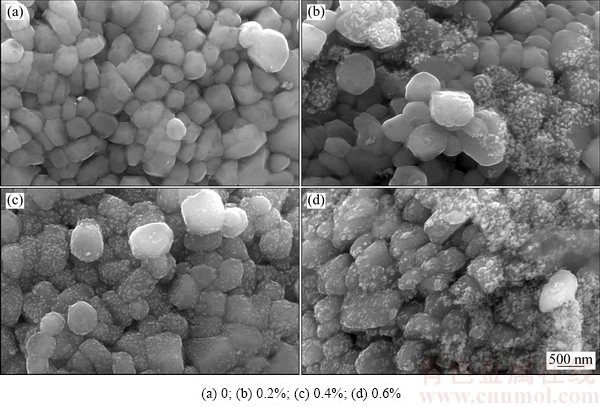
图2 氧化钇包覆LiNi0.5Co0.2Mn0.3O2的SEM像
Fig. 2 SEM images of yttrium oxide coated LiNi0.5Co0.2Mn0.3O2
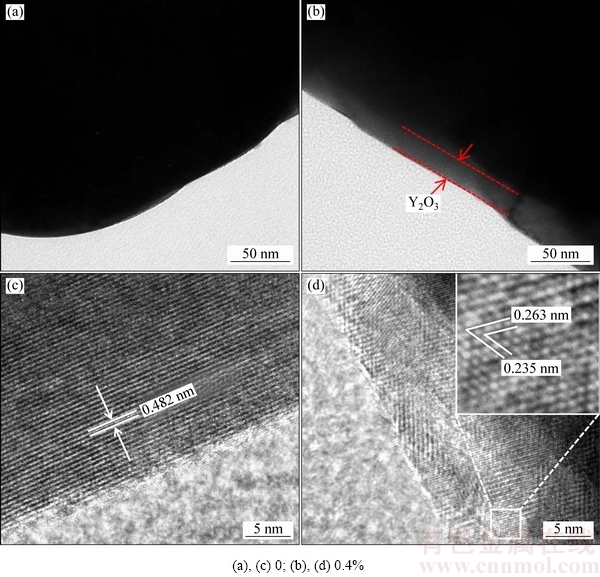
图3 氧化钇包覆LiNi0.5Co0.2Mn0.3O2的TEM像
Fig. 3 TEM images of pristine and yttrium oxide coated LiNi0.5Co0.2Mn0.3O2
图3所示为氧化钇包覆正极材料的TEM像。与未包覆样品相比(见图3(a)),经氧化钇包覆后(见图3(b)),可以看到明显的包覆层,包覆相对比较均匀,包覆层厚度在15~25 nm。图3(c)所示为未包覆样品的HRTEM像,可以看到明显的晶格条纹,晶面间距为0.482 nm,对应于LiNiO2(JCPDF#09-0063)的(003)晶面。由图3(d)氧化钇包覆LiNi0.5Co0.2Mn0.3O2的局部放大图可以看出,晶面间距为0.263和0.235nm,对应于氧化钇(JCPDF#43-1036)的(400)和(420)晶面。为了进一步确认氧化钇在正极材料表面的包覆均匀性,对氧化钇包覆LiNi0.5Co0.2Mn0.3O2的样品进行了TEM下的元素分布扫描,如图4所示。由图4中可以看出,钇元素在LiNi0.5Co0.2Mn0.3O2正极材料表面分布相对比较均匀。此外,镍、钴和锰元素在正极材料表面分布也很均匀。
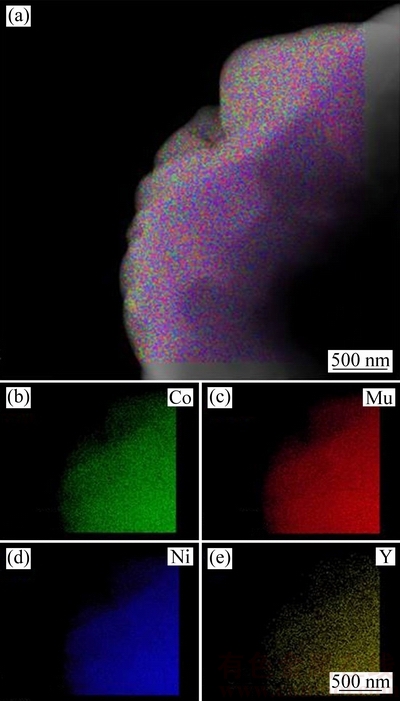
图4 氧化钇包覆LiNi0.5Co0.2Mn0.3O2的TEM像及元素分布图
Fig. 4 Typical TEM image (a) and element mappings ((b)~(e)) of yttrium oxide coated LiNi0.5Co0.2Mn0.3O2
2.3 电化学性能
图5所示为在3.0~4.4 V和3.0~4.6 V电压范围内氧化钇包覆LiNi0.5Co0.2Mn0.3O2的充放电曲线。由图5(a)中可以看出,当充放电电压为3.0~4.4 V时,0、0.2%、0.4%和0.6%氧化钇包覆LiNi0.5Co0.2Mn0.3O2的充电比容量分别为210、209、208和205 mA·h/g,对应的放电比容量分别为187、184、183和181 mA·h/g,首次充放电效率分别为89%、88%、88%和88%。由图5(b)中可以看出,当充放电电压为3.0~4.6 V时,0、0.2%、0.4%和0.6%氧化钇包覆LiNi0.5Co0.2Mn0.3O2的首次充电比容量分别为231、230、230和229 mA·h/g,放电比容量分别为205、204、203和201 mA·h/g,对应的首次充放电效率分别为89%、89%、88%和88%。随着氧化钇包覆量增加,放电比容量逐渐下降。这可能是由于氧化钇为非电化学活性物质,从而使得初始放电比容量有所下降[20]。
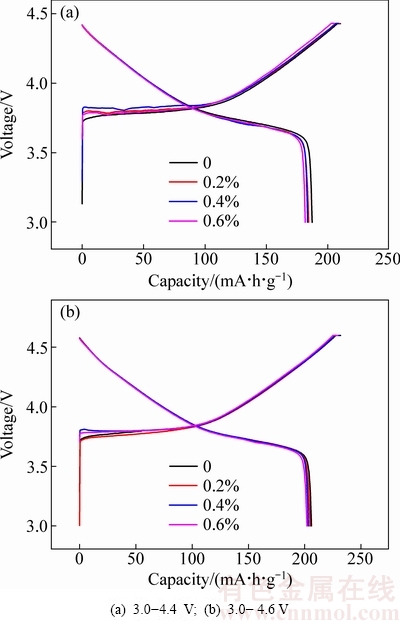
图5 氧化钇包覆LiNi0.5Co0.2Mn0.3O2的0.1C倍率下充放电曲线
Fig. 5 Charge-discharge curves of yttrium oxide coated LiNi0.5Co0.2Mn0.3O2 at rate of 0.1C
图6所示为在3.0~4.4 V和3.0~4.6 V电压范围内氧化钇包覆LiNi0.5Co0.2Mn0.3O2经过1C循环50周的循环性能图。由图6(a)可以看出,当充放电电压为3.0~ 4.4 V时,0、0.2%、0.4%和0.6%氧化钇包覆LiNi0.5Co0.2Mn0.3O2的1C首次放电比容量分别为171、170、169和168 mA·h/g,放电比容量随着氧化钇包覆量增加而逐渐减小。经过1C循环50周后,0、0.2%、0.4%和0.6%氧化钇包覆LiNi0.5Co0.2Mn0.3O2的放电比容量分别为148、157、160和154 mA·h/g,对应的容量保持率分别为86%、92%、95%和92%。当充放电电压为3.0~4.6 V时(见图6(b)),0、0.2%、0.4%和0.6%氧化钇包覆LiNi0.5Co0.2Mn0.3O2经1C循环50周的放电比容量分别为146、157、161和151 mA·h/g,对应的容量保持率分别为78%、84%、86%和81%。由此可以看出,经氧化钇包覆后,LiNi0.5Co0.2Mn0.3O2的循环稳定性普遍提高。这可能是由于氧化钇包覆降低了正极材料与电解液之间副反应,从而提高了材料的循环稳定性[21]。当包覆量为0.2%时,只有少量LiNi0.5Co0.2Mn0.3O2正极材料的表层覆盖了氧化钇,不足以完全抑制电解液与正极材料作用,因此,部分提高了材料循环性能。当包覆量为0.4%时,氧化钇包覆量可以足以减小正极材料与电解液的接触面积,因此材料表现出较好的循环稳定性。而当包覆量增加至0.6%时,氧化钇包覆量太多,可能阻碍了锂离子扩散,从而导致循环稳定性略有下降。CHEN等[22]在研究包覆量与正极材料电化学性能时得到了与本研究类似的结果。
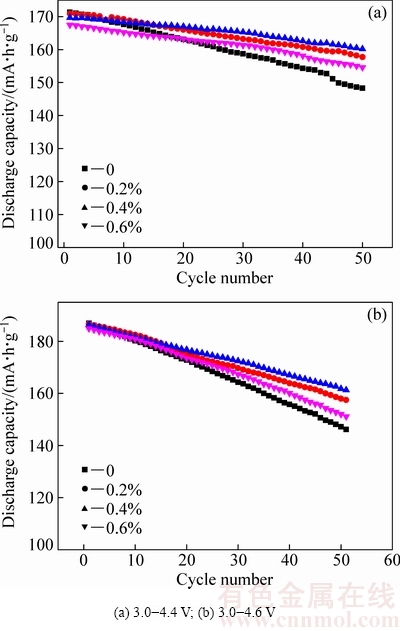
图6 氧化钇包覆LiNi0.5Co0.2Mn0.3O2 在1C倍率下循环性能
Fig. 6 Cycle performance of yttrium oxide coated LiNi0.5Co0.2Mn0.3O2 at rate of 1C
2.4 交流阻抗分析
图7所示为氧化钇包覆LiNi0.5Co0.2Mn0.3O2在3.0~4.4 V和3.0~4.6 V条件下经过1C循环50周交流阻抗图,右上角为高频区局部放大图。由图7可以看出,交流阻抗图均有一个高频区半圆、一个中频区半圆和一条低频区的斜线组成(充电截止电压为4.6 V时,由于频率测试限制,未出现低频区斜线),分别对应表面膜阻抗(Rsf)、电荷转移阻抗(Rct)和Warburg扩散阻抗。由图7中可以看出,未包覆和氧化钇包覆LiNi0.5Co0.2Mn0.3O2的表面膜电阻并未发生明显改变。在3.0~4.4V时,0、0.2%、0.4%和0.6%氧化钇包覆量LiNi0.5Co0.2Mn0.3O2的电荷转移电阻分别为122、45、29和62 Ω,在3.0~4.6 V时,0、0.2%、0.4%和0.6%氧化钇包覆量LiNi0.5Co0.2Mn0.3O2的电荷转移电阻分别为582、372、300和446 Ω。可以看出,经氧化钇包覆后,电荷转移电阻均明显减小。在循环过程中,电解液会与正极材料发生副反应,生成聚碳酸酯、LiF、LixPFx和LixPOyFz等产物,这些副反应产物是导致交流阻抗增加的重要原因[23]。表面包覆氧化钇后,电解液与正极材料的副反应减弱,副反应产物减少,这可能是电荷转移电阻明显减小的原因。氧化钇包覆量为0.4%时,电荷转移电阻最小。
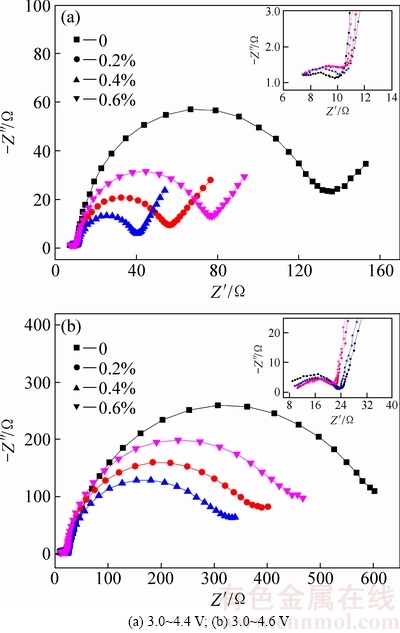
图7 氧化钇包覆LiNi0.5Co0.2Mn0.3O2组装成半电池的交流阻抗图
Fig. 7 Electrochemical impedance spectroscopy of yttrium oxide coated LiNi0.5Co0.2Mn0.3O2
2.5 循环前后循环伏安分析
图8所示为氧化钇包覆LiNi0.5Co0.2Mn0.3O2的循环伏安曲线图。由图8中可以看出,包覆与未包覆样品均只出现一对氧化还原电位峰。未包覆样品的氧化和还原电位分别位于3.90 V和3.64 V,对应Ni2+/Ni4+的氧化还原电位[24],氧化钇包覆后氧化电位与还原电位与未包覆样品接近。当循环50周后,氧化峰向高电压方向偏移,还原峰向低电压方向偏移。由图8中还可以看出,未包覆样品经过循环50周后,氧化和还原电位分别为4.01 V和3.57 V,电位差为0.44 V。氧化钇包覆样品经循环50周后,氧化和还原电位分别为3.95和3.61 V,电位差为0.34 V,可以看出,经过氧化钇包覆后,极化明显减小,这也是材料循环稳定性增加的原因。

图8 氧化钇包覆LiNi0.5Co0.2Mn0.3O2的CV曲线
Fig. 8 Cyclic voltammetry curves of yttrium oxide coated LiNi0.5Co0.2Mn0.3O2
3 结论
1) 利用氧化钇和正极材料表面带电状态不同,制备了氧化钇包覆LiNi0.5Co0.2Mn0.3O2。氧化钇以颗粒状分布在正极材料表面,包覆层厚度在15~25 nm。
2) 在3.0~4.4 V和3.0~4.6 V测试电压范围内,随着氧化钇包覆量增加,LiNi0.5Co0.2Mn0.3O2的放电比容量逐渐减小。
3) 氧化钇包覆后,LiNi0.5Co0.2Mn0.3O2的循环稳定性增加,当包覆量为0.4%时,循环稳定性最佳。
4) 氧化钇包覆后,电荷转移电阻明显减小,当包覆量为0.4%时,在3.0~4.6 V电压下,电荷转移电阻从582 Ω减小至300 Ω。
5) 经1C循环50周后,氧化钇包覆LiNi0.5Co0.2Mn0.3O2的极化明显减小,表明氧化钇包覆有效抑制了材料与电解液的作用。
REFERENCES
[1] BAK S M, HU En-yuan, ZHOU Yong-ning, YU Xi-qian, SENANAYAKE S D, CHO S J, KIM K B, CHUNG K Y, YANG Xiao-qing, NAM K W. Structural changes and thermal stability of charged LiNixMnyCozO2 cathode materials studied by combined in situ time-resolved XRD and mass spectroscopy[J]. Applied Materials and Interfaces, 2014, 6(24): 22594-22601.
[2] CHO Y H, JANG D, YOON J, KIM H, AHN T K, NAM K W, SUNG Y E, KIM W S, LEE Y S, YANG Xiao-qing, YOON W S. Thermal stability of charged LiNi0.5Co0.3Mn0.2O2 cathode for Li-ion batteries investigated by synchrotron based in situ X-ray diffraction[J]. Journal of Alloy and Compounds, 2013, 562: 219-223.
[3] SHU Jie, MA Rui, SHAO Lian-yi, SHUI Miao, WU Kai-qiang, LAO Meng-meng, WANG Dong-jie, LONG Neng-bing, REN Yuan-long. In-situ X-ray diffraction study on the structure evolutions of LiNi0.5Co0.3Mn0.2O2 in different working potential windows[J]. Journal of Power Sources, 2014, 245: 7-18.
[4] LI Yan, LIU Kai-yu, Lü Mei-yu, LAI Wei, ZHONG Jian-jian. Synthesis, characterization and electrochemical performance of AlF3-coated Li1.2(Mn0.54Ni0.16Co0.08)O2 as cathode for Li-ion battery[J]. Transaction of Nonferrous Metal Society of China, 2014, 24(11): 3534-3540.
[5] 熊利芝, 刘文萍, 吴玉先, 何泽强. 锂离子导体包覆镍锰酸锂正极材料的制备及其电化学性能[J]. 中国有色金属学报, 2015, 25(7): 1911-1919.
XIONG Li-zhi, LIU Wen-ping, WU Yu-xian, HE Ze-qiang. Preparation and electrochemical properties of lithium nickel manganese oxide cathode materials coating with lithium ion conductor[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(7): 1911-1919.
[6] TANG Hao, TAN Long, XU Jun. Synthesis and characterization of LiFePO4 coating with aluminum doped zinc oxide[J]. Transaction of Nonferrous Metals Society of China, 2013, 23(2): 451-455.
[7] WANG Ren-heng, LI Xin-hai, Wang Zhi-xing, GUO Hua-jun, HUANG Bin. PEG-combined liquid phase synthesis and electrochemical properties of carbon-coated Li3V2(PO4)3[J]. Transaction of Nonferrous Metals Society of China, 2015, 25(4): 1241-1247.
[8] 胡国荣, 刘见华, 曹雁冰, 彭忠东, 杜 柯. 分步碳包覆合成高密度LiFePO4/C复合材料[J]. 中国有色金属学报, 2012, 22(4): 1195-1200.
HU Guo-rong, Liu Jian-hua, CAO Yan-bing, PENG Zhong-dong, DU Ke. Synthesis of high density LiFePO4/C composite by stepwise carbon-coating[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(4): 1195-1200.
[9] LIU Ting, ZHAO Shi-xi, WANG Ke-zhen, NAN Ce-wen. CuO-coated LiNi0.5Co0.2Mn0.3O2 cathode material with improved cycling performance at high rates[J]. Electrochimica Acta, 2012, 85: 605-611.
[10] HU Shao-kang, CHENG Geng-hao, CHENG Ming-yao, HWANG B J, SANTHANAM RAMAN. Cycle life improvement of ZrO2-coated spherical LiNi1/3Co1/3Mn1/3O2 cathode material for lithium ion batteries[J]. Journal of Power Sources, 2009, 188(2): 564-569.
[11] BHUVANESWARI D, BABU GANGULI, KALAISELVI N. Effect of surface modifiers in improving the electrochemical behavior of LiNi0.4Mn0.4Co0.2O2 cathode[J]. Electrochimica Acta, 2013, 109: 684-693.
[12] 袁 好, 王先友, 胡 亮, 杨秀康, 舒洪波. 纳米CuO表面包覆对Li1.13[Ni0.5Mn0.5]0.87O2正极材料电化学性能的影响[J]. 中国有色金属学报, 2016, 26(9): 1929-1934.
YUAN Hao, WANG Xian-you, HU Liang, YANG Xiu-kang, SHU Hong-bo. Effects of CuO-coating on electrochemical performance of Li1.13[Ni0.5Mn0.5]0.87O2 cathode material[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(9): 1929-1934.
[13] 李 卫, 田文怀, 其 鲁. LiMg0.03(Ni0.77Co0.1Mn0.1)O2×B2O3 球形高镍材料的制备与性能[J]. 中国有色金属学报, 2015, 25(10): 2752-2759.
LI Wei, T|IAN Wen-huai, QI Lu. Preparation and performance of spherical high nickel material LiMg0.03(Ni0.77Co0.1Mn0.1)O2× B2O3[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(10): 2752-2759.
[14] 朱继平, 张 胜, 辛智强, 许全保, 苏 徽. 改性正极材料的合成及其电化学性能[J]. 中国有色金属学报, 2014, 24(4): 974-980.
ZHU Ji-ping, ZHANG Sheng, XIN Zhi-qiang, XU Quan-bao, SU Hui. Synthesis and electrochemical properties of modified LiNi1/3Co1/3Mn1/3O2 cathode materials[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(4): 974-980.
[15] 赵世玺, 李颖达, 丁 浩, 李宝华, 南策文. 纳米Al2O3 包覆LiFePO4/C 正极材料的结构和电化学性能[J]. 无机材料学报, 2013, 28(11): 1265-1269.
ZHAO Shi-xi, LI Ying-da, DING Hao, LI Bao-hua, NAN Ce-wen. Structure and electrochemical performance of LiFePO4/C cathode materials coated with nano Al2O3 for lithium ion battery[J]. Journal of Inorganic Materials, 2013, 28(11): 1265-1269.
[16] WU Feng. WANG Meng, SU Ye-feng, CHEN Shi. Surface modification of LiCo1/3Ni1/3Mn1/3O2 with Y2O3 for lithium-ion battery[J]. Journal of Power Sources, 2009, 189(1): 743-747.
[17] JU Bo-wei, WANG Xian-you, WU Chun, WEI Qi-liang, YANG Xiu-kang, SHU Hong-bo, BAI Yan-song. Excellent cycling stability of spherical spinel LiMn2O4 by Y2O3 coating for lithium-ion batteries[J]. Journal of Solid State Electrochemistry, 2014, 18(1): 115-123.
[18] 王 萌, 吴 锋, 苏岳锋, 陈 实. Y2O3 包覆LiNi1/3Co1/3Mn1/3O2的电化学性能[J]. 物理化学学报, 2008, 24(7): 1175-1179.
WANG Meng, WU Feng, SU Yue-feng, CHEN Shi. Electrochemical characteristics of Y2O3 coated LiNi1/3Co1/3- Mn1/3O2[J]. Acta Physico-chimica Sinica, 2008, 24(7): 1175-1179.
[19] LU Chao, WU Hao, ZHANG Yun, LIU Heng, CHEN Bao-jun, WU Nai-teng, WANG Sen. Cerium fluoride coated layered oxide Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials with improved electrochemical performance for lithium ion batteries[J]. Journal of Power Sources, 2014, 267: 682-691.
[20] ZUO Da-xian, TIAN Guang-lei, CHEN Da, SHEN Hang-yan, Lü Chun-jun, SHU Kang-ying, ZHOU Yu-fang. Comparative study of Al2O3-coated LiCoO2 electrode derived from different Al precursors: uniformity, microstructure and electrochemical properties[J]. Electrochimica Acta, 2015, 178: 447-457.
[21] JU Bo-wei, WANG Xian-you, WU Chun, YANG Xiu-kang, SHU Hong-bo, BAI Yan-song, WEN Wei-cheng, YI Xin. Electrochemical performance of the grapheme/Y2O3/LiMn2O4 hybrid as cathode for lithium ion battery[J]. Journal of Alloy and Compounds, 2014, 584: 454-460.
[22] CHEN Quan-qi, WANG Yao-bin, ZHANG Ting-ting, YIN Wu-mei, YANG Jian-wen, WANG Xian-you. Electrochemical performance of LaF3-coated LiMn2O4 cathode materials for lithium ion batteries[J]. Electrochimica Acta, 22, 83: 65-72.
[23] ARUMUGAM D, PARUTHIMAL KALAIGNAN G. Synthesis and electrochemical characterization of nano-CeO2 coated nanostructure LiMn2O4 cathode materials for rechargeable lithium batteries[J]. Electrochimica Acta. 2010, 55(28): 8709-8716.
[24] LIU Kun, YANG Gao-li, DONG Yue, SHI Ting, CHEN Li. Enhanced cycling stability and rate performance of Li[Ni0.5Co0.2Mn0.3O2] by CeO2 coating at high cut-off voltage[J]. Journal of Power Sources, 2015, 281: 370-377.
Structure and electrochemical performance of yttrium oxide coated LiNi0.5Co0.2Mn0.3O2
XU Jun-na1, 2, CHEN Xiao-qing2, GAO Xiong3, ZHOU You-yuan3,
XIAO Ke-song4, HUANG Cheng-huan3, XI Xiao-ming4
(1. Kingray New Materials Science and Technology Co., Ltd., Changsha 410012, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
3. Hunan Changyuan LiCo Co., Ltd., Changsha 410205, China;
4. Changsha Research Institute of Mining and Metallurgy Co., Ltd., Changsha 410012, China)
Abstract: Yttrium oxide coated LiNi0.5Co0.2Mn0.3O2 was successfully prepared based on the different surface potentials between the cathode material and yttrium oxide using yttrium oxide sol as coating precursor. The crystal structure, morphology and electrochemical properties of uncoated and yttrium oxide coated LiNi0.5Co0.2Mn0.3O2 were investigated by X-ray diffractometry(XRD), scanning electron microscopy(SEM), transmission electron microscopy(TEM) and electrochemical measurements. The results indicate that the crystal structure is not affected by yttrium oxide coating, the yttrium oxide particles homogenously distribute on the surface of cathode materials and the thickness of coating layer is 15-25 nm. Comparing with the uncoated LiNi0.5Co0.2Mn0.3O2, the cycle performance of the cathode material improves after yttrium oxide coating and the optimum coating amount is 0.4%. The charge transfer resistance and the polarization are suppressed after coating during the charge-discharge processes.
Key words: yttrium oxide; surface coating; LiNi0.5Co0.2Mn0.3O2; electrochemical performance
Foundation item: Project(2016YFB0100400) supported by the National Key Research and Development Plans of China; Project(51474037) supported by the National Natural Science Foundation of China
Received date: 2016-12-21; Accepted date: 2017-05-12
Corresponding author: XI Xiao-ming; Tel: +86-731-88657399; E-mail: xixiaoming@vip.163.com
(编辑 李艳红)
基金项目:国家重点研发计划资助项目(2016YFB0100400);国家自然科学基金资助项目(51474037)
收稿日期:2016-12-21;修订日期:2017-05-12
通信作者:习小明,教授,博士;电话:0731-88657399;E-mail:xixiaoming@vip.163.com
摘 要:以氧化钇溶胶为包覆前驱物,利用氧化钇和正极材料表面带电状态不同制备氧化钇包覆LiNi0.5Co0.2Mn0.3O2。采用X射线衍射仪(XRD)、扫描电镜(SEM)、透射电镜(TEM)及电化学测试等手段对LiNi0.5Co0.2Mn0.3O2包覆前后的物相结构、表面形貌及电化学性能进行研究。结果表明:氧化钇包覆并未影响LiNi0.5Co0.2Mn0.3O2的晶体结构,氧化钇以颗粒状分布在正极材料表面,氧化钇包覆层厚度在15~25 nm,氧化钇在正极材料表面分布比较均匀。与未包覆LiNi0.5Co0.2Mn0.3O2相比,氧化钇包覆后,材料在高电压下的循环稳定性有所提高,最佳包覆量为0.4%。氧化钇包覆有效降低材料在充放电过程中的极化和电荷转移电阻。
[5] 熊利芝, 刘文萍, 吴玉先, 何泽强. 锂离子导体包覆镍锰酸锂正极材料的制备及其电化学性能[J]. 中国有色金属学报, 2015, 25(7): 1911-1919.
[8] 胡国荣, 刘见华, 曹雁冰, 彭忠东, 杜 柯. 分步碳包覆合成高密度LiFePO4/C复合材料[J]. 中国有色金属学报, 2012, 22(4): 1195-1200.
[14] 朱继平, 张 胜, 辛智强, 许全保, 苏 徽. 改性正极材料的合成及其电化学性能[J]. 中国有色金属学报, 2014, 24(4): 974-980.
[15] 赵世玺, 李颖达, 丁 浩, 李宝华, 南策文. 纳米Al2O3 包覆LiFePO4/C 正极材料的结构和电化学性能[J]. 无机材料学报, 2013, 28(11): 1265-1269.
[18] 王 萌, 吴 锋, 苏岳锋, 陈 实. Y2O3 包覆LiNi1/3Co1/3Mn1/3O2的电化学性能[J]. 物理化学学报, 2008, 24(7): 1175-1179.


