J. Cent. South Univ. (2017) 24: 754-765
DOI: 10.1007/s11771-017-3477-x
Preparation and characterization of highly photocatalytic active hierarchical BiOX (X=Cl, Br, I) microflowers for rhodamine B degradation with kinetic modelling studies
GU Ying-ying(古映莹), ZHAO Li(赵莉), YANG Ming-yang(杨明阳), XIONG Yi-qiu(熊意求),
WU Zhe(吴喆), ZHOU Min-jia(周敏嘉), YAN Jun(颜军)
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Abstract:
The hierarchical BiOX (X=Cl, Br, I) microflowers were successfully synthesized via simple precipitation method at 160 °C for 24 h and characterized by XRD, SEM, TEM, UV-vis DRS and N2 adsorption-desorption techniques. The as-prepared samples were pure phases and of microflowers composed of nanosheets which intercrossed with each other. The specific surface areas were about 22.9, 17.3 and 16.2 m2/g for BiOCl, BiOBr and BiOI, respectively. The photocatalytic activities of BiOX powers were evaluated by RhB degradation under UV-vis light irradiation in the order of BiOCl > BiOBr > BiOI. Also, the kinetics of RhB degradation over BiOI was selectively investigated, demonstrating that the kinetics of RhB degradation follows apparent first-order kinetics and fits the Langmuir-Hinshelwood model.
Key words:
microstructure; semiconductors; photocatalysis; BiOX;
1 Introduction
Degrading organic compounds and splitting water into hydrogen by photocatalysis is considered as a safe, cost-effective, and “green” technology, which has received global attention for environmental protection and clean energy production [1-9]. Among the semiconductor photocatalysts, TiO2 with easy availability and non-toxicity becomes most popular [10-12]. However, their low quantum yields and the fact that pure TiO2 can only be activated by UV light prevent their common application [13, 14]. In order to effectively utilize solar energy, new strategies on design and synthesis of photocatalysts were explored, and the development of new and efficient visible-light-driven photocatalysts remains a major task from the practical use and commercial viewpoints [15]. Since nanostructured BiOCl was reported by ZHANG et al [16] as a photocatalyst and demonstrated higher photocatalytic activity than TiO2 (P25, Degussa) on photocatalytic degradation of methyl orange (MO) dye [16]. BiOX (X= Cl, Br, I), as one of the important main group multicomponent V–VI–VII semiconductors, has been proved to be effective for photocatalytic activities under visible light irradiation owing to their unique layered structures and high chemical stability [17-19]. As well known, BiOX has a tetragonal PbFCl-type structure consisting of [X-Bi-O-Bi-X] layers stacked one above the other by nonbonding van der Waals interaction through the halide atoms along c-axis [20, 21]. The internal static electric fields perpendicular to [Bi2O2]2+ and the anionic halogen layers are induced through polarization of atoms, leading to effective separation of the photo-induced electron–hole pairs and then augmenting photocatalytic properties of the catalysts. Further, the band gaps of BiOCl, BiOBr and BiOI (around 3.22, 2.64 and 1.77 eV, respectively) decrease with the decreasing electronegativity of halogen elements in compounds, from which the conduction-band minimum and the valence-band maximum are derived from the Bi 6pz and M np orbitals (M=Cl, Br, I) [22, 23]. And the indirect-transition band gap nature of BiOX has prohibited the electron-hole recombination successfully [24]. Apart from crystal structure, phase, size, morphology, dimensionality and specific surface area related to activity sites of photocatalytic materials play decisive roles in photocatalytic properties [25-28].
Numerous BiOX with different morphologies, such as nanofibers [29], nanosheets [30], nanoparticles [31], nanoplates [21], nanobelts and nanotubes [32], have been developed by different preparative approaches including electro-spinning, hydrolytic, hydrothermal, solvothermal methods and so on. But these reported structures of BiOX still have a few deficiencies in photocatalysis. The nanoparticles and nanoplates are too small to separate from the photocatalytic reaction solution to recycle, while the active surface area of the catalysts is reduced obviously owing to the tendency to aggregate during aging, which attenuates the photocatalytic performance. Therefore, the 3D hierarchical flower-like spheres [33-39] assembled with nanostructured building blocks have drawn much attention recently because the unique and novel morphology possesses larger specific surface area and exposes more active sites than nanoparticles and 2D micro/nanostructures. For instance, ZHU et al [40] reported that self-assembled 3D BiOCl hierarchitectures exhibited a higher photocatalytic activity than that of BiOCl nanofibers [29]. HU et al [41] fabricated BiOI hierarchical microspheres with ethylene glycol as the solvent, which exhibited much better abilities in both adsorption and photocatalytic efficiency than that of BiOI nanosheets fabricated with H2O as the solvent. However, most of the previously reported hierarchical structures are synthesized by independently altered methods and little attention is paid on the methodical fine tuning effect of halide ions on these hierarchical microspheres. Only a few systematic examples were described, such as LI et al [42] reported a facile microwave irradiation method to fabricate BiOX nanostructures in mannitol solution, and the morphology and size of BiOX nanostructures could be readily tailored by adjusting synthetic parameters. Also, ZHANG et al [22] and CHEN et al [43] reported another two BiOX systems with flower-like hierarchical structures respectively, and all systems revealed highly photocatalytic properties. In addition, BiOX are well-known photocatalysts, and the reactant adsorption on the catalyst surface is believed to generally follow the Langmuir-Hinshelwood (L-H) model in the system. [44-47] While the kinetic modelling could further benefit the design and application of new hierarchical structures, so it is valuable to study the photocatalytic dynamics of hierarchical structures BiOX.
In this regards, herein we report the preparation of pure phase BiOX photocatalysts. The as-prepared powders were microflowers with hierarchical superstructures. Their photocatalytic performances were investigated through degrading RhB under UV-vis light irradiation. Further studies demonstrated that the high photocatalytic activities of BiOX were correlated with their specific surface areas, which were supported by a series of joint techniques. The photocatalytic kinetic properties of BiOI were also conducted preliminarily for the first time. The reaction rate constants were consistent with the order of photocatalytic activities of BiOX. Meanwhile the experimental results indicate that RhB degradation using microflowers BiOI is well-described by the L-H model and fitted apparent first-order kinetics.
2 Experimental
2.1 Materials
Bismuth nitrate pentahydrate (Bi(NO3)3·5H2O), hexadecyl trimethyl ammonium chloride (CTAC), cetyl trimethyl ammonium bromide (CTAB), potassium iodide (KI), ethylene glycol (EG), rhodamine-B dye (N,N,N’,N’-tetraethyl rhodamine, RhB) and ethanol were obtained from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. All of the chemicals reagents used in this experiment were analytical grade without further purification. Deionized water was used throughout this work.
2.2 Preparation of BiOX(X=Cl, Br,I) powders
The self-assembled hierarchical microflowers of BiOX powders were synthesized via solvothermal processes as follows. In a typical experimental procedure [48], 4 mmol of (Bi(NO3)3·5H2O) (1.98 g) was dissolved in 80 mL of EG solutions containing stoichiometric amounts of CTAC (1.32 g) and CTAB (1.46 g) respectively, with the Bi to X molar ratio of 1. The mixture solutions were stirred vigorously for 1 h until the pH reached about 3.5, and subsequently transferred into a 100 mL Teflon-lined stainless steel autoclave. The autoclave was allowed to be heated up to 160 °C for 24 h under spontaneous pressure. In the courses of synthesizing BiOCl and BiOBr, CTAC and CTAB were not only served as halogen (Cl, Br) sources but also acted as structure-directing agents to control morphology and size. Different from BiOCl and BiOBr, BiOI flowerlike hierarchical structures were successfully obtained by a template-free solvothermal method and KI was used as iodine source. 1 mmol Bi(NO3)3·5H2O was dissolved in 17 mL of ethanol to get solution A. Solution B was prepared by dissolving 1 mmol KI in 17 mL of deionized water. Then solution A was added slowly to solution B with continuous stirring to form brick red precipitate finally. The pH of the suspension was about 3. The mixture also was stirred for 1 h and then poured into a 50 mL Teflon-lined stainless steel autoclave heated at 160 °C for 24h. The products were collected by centrifugation, subsequently washed with deionized water and ethanol for several times, and dried at 80°C for 5 h in the air.
2.3 Characterization
The XRD data were recorded by using a Rigaku D/Max 2500 X-ray diffractometer with a Cu Kα radiation source of λ=0.15406 nm, voltage of 40 kV, current of 250 mA, and a scanning rate of 8.0 (°)/min. The morphologies were investigated by a field emission scanning electron microscope (FE-SEM) (HITACHI S-4800). Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) characterizations were taken over in a JEOLJEM-2010 (JEOL Ltd., Tokyo, Japan) at 200 kV. The ultraviolet-visible diffuse reflectance spectroscopy (UV-vis DRS) was observed by using a UV-visible spectrophotometer (UV-2600; Shimadzu Corp., Japan). BaSO4 was selected as the reference. Then the reflectance spectra were converted to the absorption spectra by the Kubelka-Munk method [49]. N2 adsorption-desorption isotherms were recorded at 77 K using a V-Sorb 2800P instrument after the sample had been degassed in a vacuum at 180 °C for 3 h. The Brunauer–Emmett–Teller (BET) surface areas were calculated by using the BET equation from the adsorption data (P/P0=0.06-0.3). The pore diameter distribution was estimated by the Barrett–Joyner– Halenda (BJH) method.
2.4 Photocatalytic activity evaluation
As a model organic pollutant, RhB was chosen to evaluate the photocatalytic activities of the as- synthesized BiOX under UV-vis light irradiation. The optical system used for the photocatalytic degradation of RhB consisted of a metal halogen lamp (400 W) acted as a photo source and a cooling jacket of the reactor with a circulating water flow to maintain the experimental temperature at room temperature. The typical processes were performed in the air as follows. In each experiment, 0.05 g of sample was dispersed in a glass container containing 50 mL of RhB aqueous solution with an initial concentration of 15 mg/L. The suspension was stirred in the dark for 30 min to reach adsorption- desorption equilibrium prior to UV-vis light irradiation. Then the solution was exposed to radiation. At the same time, the same RhB solution without catalyst as a reference also was irradiated. At each defined irradiation time interval, 4 mL of the suspension was taken out, and then was centrifuged at 4500 r/min for 10 min to remove the photocatalyst particles. The RhB concentration was further analyzed in a Shimadzu UV-2600 UV-vis spectrophotometer, and the absorbance at 554 nm was detected to monitor the photocatalytic degradation. The photocatalytic removal rate D was calculated by the formula:
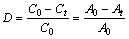
where A0 and C0 are the corresponding initial absorbance and concentration before irradiation while At and Ct are the absorbance and concentration at time t of irradiation.
3 Results and discussion
3.1 Phase structure analysis
The XRD patterns of as-prepared BiOX (X=Cl, Br, I) are shown in Fig. 1. All the diffraction peaks can be perfectly indexed to the tetragonal phase of BiOCl (JCPDS 06-0249; cell constants a=b=3.891 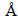 , c= 7.369
, c= 7.369 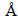 ), BiOBr (JCPDS 09-0393; cell constants a=b= 3.926
), BiOBr (JCPDS 09-0393; cell constants a=b= 3.926 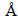 , c=8.103
, c=8.103 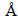 ) and BiOI (JCPDS 10-0445; cell constants a=b=3.994
) and BiOI (JCPDS 10-0445; cell constants a=b=3.994 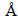 , c=9.149
, c=9.149 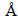 ), with space groups of P4/nmm, respectively. No diffraction peaks of other phase can be observed, demonstrating that all of the products are phase-pure. As known, a rise in intensity of a particular diffraction peak implies a better exposure of plane that causes the reflection [38]. For the BiOCl crystal phase, it can be found that the intensity of (110) diffraction peak at 2θ of 32.54° is stronger than that of other diffraction peaks, which indicates a preferred growth along the (110) crystallographic direction which is perpendicular to the c axis. In the same way, the relatively strong diffraction peaks at around 2θ of 32.22° and 29.60° represent the (110) plane of BiOBr crystal and (102) plane of BiOI crystal, respectively. This means that the BiOBr should favour to grow along the (110) orientation and the BiOI should preferentially grow along the (102) orientation during the synthetic process. And the d-spacings analyzed through XRD data are 0.275, 0.278 and 0.300 nm, respectively, corresponding to the (110) plane of BiOCl, (110) plane of BiOBr and (102) plane of BiOI. These results are further demonstrated below by HRTEM. In addition, as can be seen from the XRD patterns, the diffraction peaks of BiOI are sharper and stronger compared with the diffraction peaks of BiOCl and BiOBr, which indicates the highest crystallinity among the three kinds of samples. These can also be evidenced by the following SEM and TEM analysis results.
), with space groups of P4/nmm, respectively. No diffraction peaks of other phase can be observed, demonstrating that all of the products are phase-pure. As known, a rise in intensity of a particular diffraction peak implies a better exposure of plane that causes the reflection [38]. For the BiOCl crystal phase, it can be found that the intensity of (110) diffraction peak at 2θ of 32.54° is stronger than that of other diffraction peaks, which indicates a preferred growth along the (110) crystallographic direction which is perpendicular to the c axis. In the same way, the relatively strong diffraction peaks at around 2θ of 32.22° and 29.60° represent the (110) plane of BiOBr crystal and (102) plane of BiOI crystal, respectively. This means that the BiOBr should favour to grow along the (110) orientation and the BiOI should preferentially grow along the (102) orientation during the synthetic process. And the d-spacings analyzed through XRD data are 0.275, 0.278 and 0.300 nm, respectively, corresponding to the (110) plane of BiOCl, (110) plane of BiOBr and (102) plane of BiOI. These results are further demonstrated below by HRTEM. In addition, as can be seen from the XRD patterns, the diffraction peaks of BiOI are sharper and stronger compared with the diffraction peaks of BiOCl and BiOBr, which indicates the highest crystallinity among the three kinds of samples. These can also be evidenced by the following SEM and TEM analysis results.
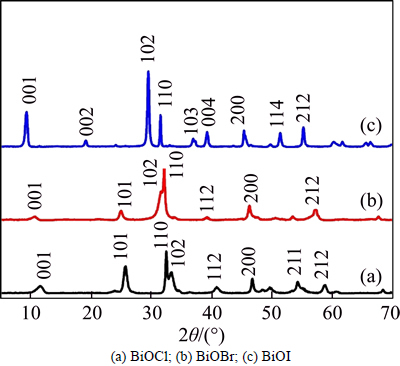
Fig. 1 XRD patterns of as-prepared BiOX (X=Cl, Br, I) powers:
3.2 Morphology analysis
Figures 2(a)-(c) are low-magnification SEM images of BiOX samples, revealing that all the BiOX powders consist of hierarchical microflowers. Compared with BiOCl and BiOBr, the nanosheets on the BiOI surface accumulate tighter, which may lead to the smaller specific surface area of BiOI. As shown in Fig. 2(a), the sizes of BiOCl are not uniform and their diameters are about in the range of 1-2 μm, while BiOBr and BIOI in Figs. 2(b) and (c) possess relatively uniform sizes, and the diameters are about 1.5 μm for BiOBr and 5 μm for BiOI. These results consist with the previously reports on fabrication BiOX nanostructures under similar conditions [18]. The type of halogen sources is not crucial in forming hierarchical microflowers, and the reaction temperature, reaction time and pH value can affect the morphology and size of BiOX [50-52]. Further, higher magnifications of the images are shown in Figs. 2(a′)- (c′), demonstrating that the BiOX microflowers are assembled by numerous smaller nanosheets. These nanosheets stack and intercross with one another and then form porous structures. These pores between the nanosheets may amplify the surface areas of samples and expose more active sites resulting in a higher photocatalytic activity.
In order to obtain more detailed crystal structures of BiOX, TEM and HRTEM were carried out and the results are shown in Fig. 3. As shown in Figs. 3(a) and (b), the structures of BiOCl and BiOBr are integrated hierarchical microflowers. And the nanosheets of about several nanometers in thickness are observed on the edge of the microflowers. However, under the same magnification (see Fig. 3(c)), only part of a BiOI microflowers can be observed due to its larger size. It can also be found that a mass of irregular nanosheets stack closely on the surface of BiOI. All of these are similar to the SEM observation. Particularly, many gaps of BiOCl microflowers are revealed in Fig. 3(a) and this may be caused by looser accumulation of the nanosheets, which may increase the specific surface area of BiOCl. The edges of BiOX nanosheets were further investigated by HRTEM. As shown in Figs. 3(a′)-(c′), the single crystalline nature of the nanosheets in the microflowers is revealed. What is more, the HRTEM images show clearly the lattice fringes with lattice spacings of about 0.277, 0.278 and 0.310 nm, which are well matched with the crystallographic plane of BiOCl (110), BiOBr (110) and BiOI (102), respectively. These results are also in accordance with the XRD analysis of BiOX, as listed in Table 1.
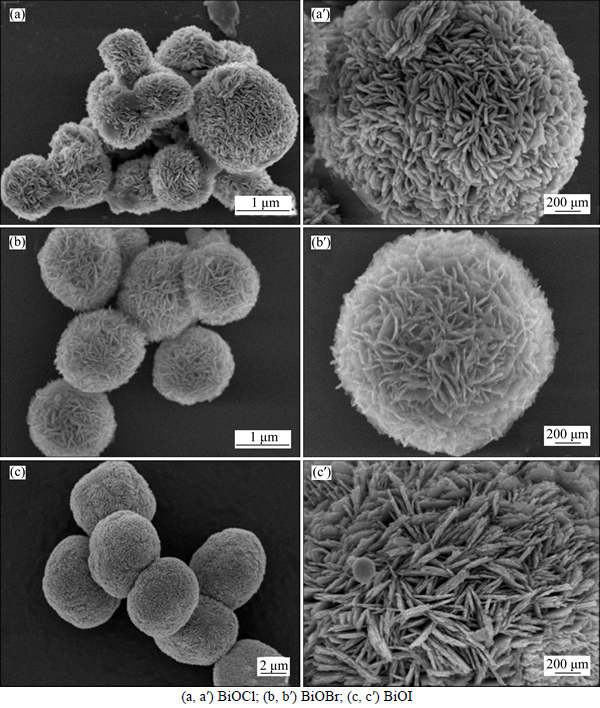
Fig. 2 SEM images of as-prepared BiOX (X=Cl, Br, I) powders:
Fig. 3 TEM (a, b, c) and HRTEM (a′, b′, c′) images of as-prepared BiOX:
3.3 Optical property analysis
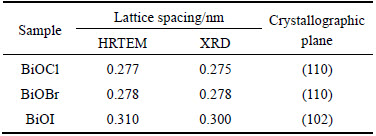
The optical properties of BiOX (X=Cl, Br, I) distinguished by diffuse reflectance spectra (DRS) are shown in Fig. 4. Figure 4(a) shows that the UV-vis absorption edges of the BiOX samples have a clear indication of red-shift along with the increasing atomic number of X. The absorption edge of BiOCl is 369 nm which lies in the near-UV regions, while BiOBr and BiOI powers have intense absorption edges at about 420 and 654 nm in the visible light region, indicating that the samples are able to take full advantage of visible light. These results are also consistent with their respective colours as white, light yellow and carmine red of BiOCl, BiOBr and BiOI solid powders. Moreover, as crystalline semiconductors with indirect transition, the band gaps of BiOX are calculated by the formula αhv=A(hv-Eg)2, where α, h, v, Eg and A are the absorption coefficient, Planck constant, light frequency, band gap energy and a constant, respectively [16, 53, 54]. Figure 4(b) depicts the plots of (Ahν)1/2 versus the photon energy (hν) for the samples. The intercept of the tangent to the x-axis, as a good approximation, is used to estimate the band gap energies of BiOCl, BiOBr and BiOI powers, which are 3.24, 2.67 and 1.84 eV, respectively. This observed band gap energies are close to the previous reports [22]. It is seen clearly that the band gap energies of BiOX can be fine tuned by varying morphologies and they decrease gradually along with increasing atomic number of X.
Table 1 HRTEM and XRD results of lattice spacing of BiOX
3.4 N2 adsorption-desorption analysis
The adsorption ability of photocatalysts for a specific pollutant is a noticeable factor in affecting photocatalytic property. The specific surface areas and porosity of the BiOX samples were measured by nitrogen sorption. Figure 5 shows the N2 adsorption– desorption isotherms of as-prepared BiOX microflowers and the insets depict the corresponding pore-size distributions. As shown, the isotherms of BiOCl and BiOBr samples display typical type-IV isotherms with distinct hysteresis loops observed in the range of 0.5- 1.0 P/P0, indicating the presence of mesoporous structure (2–50 nm in size) [55]. While the isotherm of BiOI shows the type II adsorption-desorption isotherm, in which the weak adsorption-desorption hysteresis indicates monolayer absorption and smaller pore size [56]. Moreover, the high adsorption at high relative pressure (near P/P0=1.0) indicates the existence of macropores (> 50 nm) of BiOX in all of the insets [55]. Furthermore, the foregoing results are confirmed by the corresponding pore-size distributions of BiOX estimated based on the Barrett-Joyner-Halenda (BJH) method (see insets in Fig. 5). The pore-size distributions indicate that both the BiOCl and BiOBr samples have small mesopores and large mesopores, while BiOI samples contain mainly small mesopores, as well as all of them have macropores. The smaller pores may arise from the primary BiOX nanosheets growth process, whereas the larger pores and macropores can be attributed to the space between the intercrossed nanosheets [57, 58] and their existence can be observed in the SEM images. The Brunauer-Emmett-Teller (BET) specific surface areas and pore volumes of as-prepared BiOX powders were calculated and are summarized in Table 2. It is found that BiOBr and BiOI have BET surface areas of 17.3 and 16.2 m2/g, respectively, while BiOCl has the relatively large surface area of 22.9 m2/g. Obviously, the specific surface areas of BiOX decrease with increasing atomic number of X, which is the same to the pore volume. The large surface area of BiOCl should be ascribed to the loose agglomeration between nanosheets, which can be observed from the TEM images in Fig. 3(a). Since the large surface area may expose more active sites and play an important role in the adsorption process, it is very reasonable to expect that BiOCl materials have higher adsorption capacity and photocatalytic activity for the degradation of RhB in water.
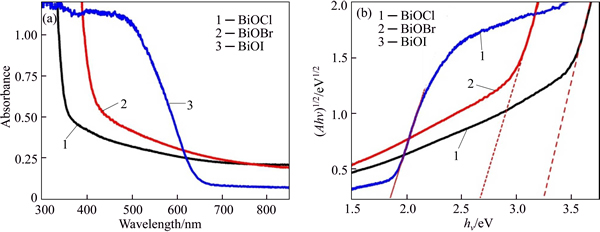
Fig. 4 UV-vis diffuse reflectance spectra (a) and corresponding (Ahv)1/2-hv plots (b) of BiOX (X=Cl, Br, I)
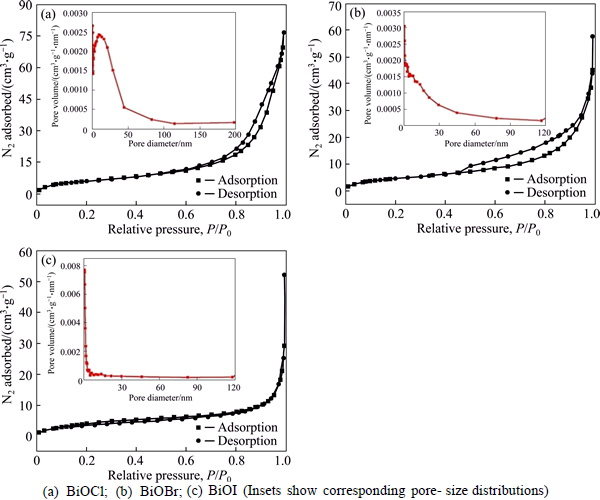
Fig. 5 N2 adsorption-desorption isotherms of BiOX powers:
Table 2 Textural and photocatalytic properties of BiOX (X=Cl, Br, I)

3.5 Photocatalytic activity of as-prepared BiOX
The photocatalytic activities of the BiOX (X=Cl, Br, I) samples were measured on the degradation of RhB as the model pollutant with an initial concentration of 15 mg/L under UV-vis light illumination. The temporal evolutions of the spectral changes of the RhB with irradiation time over BiOCl, BiOBr, and BiOI microflowers are illustrated in Figs. 6(a)-(c). It can be seen that the characteristic absorption peak of RhB at λ=554 nm diminishes gradually as the exposure time increases in the presence of the photocatalysts. Before irradiation, the adsorption-desorption equilibrium between RhB and catalysts was quickly established within 30 min then degradation proceeded at t=0. The RhB adsorption rates are estimated to be 93.3%, 88.5%, and 80.3% for BiOCl, BiOBr, and BiOI, respectively. BiOX hierarchical microflowers have good adsorption capacity, the order of which is BiOCl>BiOBr>BiOI. This is consistent well with the level of their surface areas and photocatalytic activities. Figure 6(d) presents the variation of the RhB concentration (C/C0) with irradiation time over different BiOX photocatalysts, where C0 is the initial concentration of RhB and C is the concentration of RhB at t time. As a comparison, the photodegradation of RhB without (blank) photocatalyst was performed under identical conditions. As displayed in Fig. 6(d), the blank experiment with no distinct changes confirms that direct photolysis of RhB could be neglected under UV-vis light irradiation. Within 6 min irradiation, the photocatalytic removal rates D are about 99.4% and 93.8% with the existence of BiOCl and BiOBr, respectively, while the removal rate is 96.2% in 30 min in the BiOI photocatalyst dispersion as shown in Table 2. Therefore, it is demonstrated that BiOCl exhibits the highest photocatalytic activity among the three kinds of BiOX samples. In contrast, all the hierarchical BiOX microspheres show much better photocatalytic activity than those of P25 TiO2 in the similar reaction conditions [59, 60].
Such a significant photocatalytic performance is mainly attributed to its larger pore volume, higher adsorption capacity and the efficient charge separation [61]. The large specific surface area is considered as one of the most important factors for the photocatalytic degradation of organic dye, because the higher specific surface area can expose more active sites and reflect higher adsorption capacity. It is generally accepted that the photocatalytic reaction has been preceded mainly by photocatalytic oxidation process. The primary reactive species, involving in the photocatalytic reaction, exist in the surface of the catalysts. Many researches documented that abundant reaction sites on catalysts surface are greatly beneficial to the photocatalytic degradation [62, 63]. On the basis of photocatalytic data, it is believed that high adsorption property of catalysts for RhB dye can enhance photodegradation efficiency, which is consistent with the results reported by WANG et al [64] and XU et al [65]. Besides, as BiOCl cannot be completely excited to produce reactive radicals for the RhB degradation under visible light irradiation (λ>420 nm) because of its large band gap [21, 22, 66, 67], the degradation process of BiOCl includes both photocatalytic pathway and photosensitization pathway under UV-vis light irradiation, which improves the photocatalytic performance. Additionally, as shown in Fig. 6, there is very big difference in the photocatalytic performance between BiOBr and BiOI, although their specific surface areas are similar. The relatively low photocatalytic performance of BiOI may be caused by the smaller band gap (1.84 eV) of BiOI which promotes the recombination probability of photogenerated electron–hole pairs. Also, the obvious carmine red of BiOI may weaken the intensity of light through the depth of the reaction solution, which could decrease the photocatalytic activity.
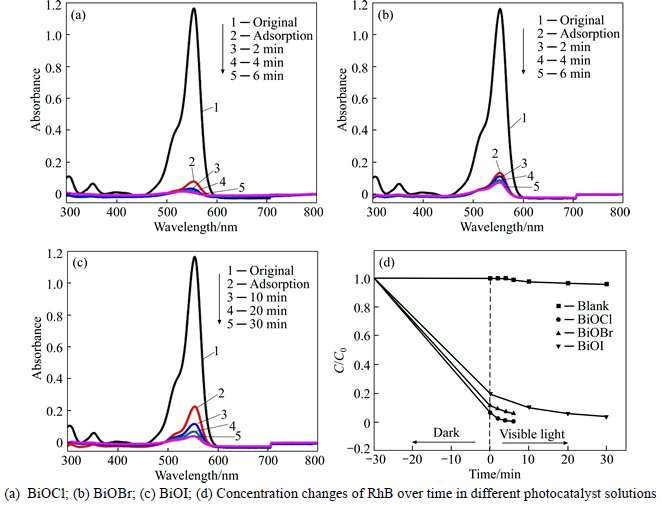
Fig. 6 Temporal evolution of UV-vis spectra during photodegradation of RhB mediated by BiOX photocatalysts:
3.6 Kinetics analysis
To examine more accurately the photocatalytic performance of BiOX, the reaction kinetics of RhB degradation over the samples under UV-vis light irradiation was investigated. The photocatalytic activity of samples can also be quantitatively evaluated by the apparent reaction rate constants. As well known, when the concentration of the original solution is low, the photocatalytic reaction can conform to apparent first- order kinetics model. The equation is:
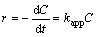 (1)
(1)
with setting t=0, C=C0, the above equation is converted to
 (2)
(2)
where C0 and C are the concentrations of RhB in aqueous solution at time 0 (the time to get adsorption–desorption equilibrium) and t, respectively, and r and kapp are the degradation rate and the apparent reaction rate constant [50, 68]. In our work, through the analysis of photodegradation data, it is not difficult to find that the RhB degradation over BiOX could well follow first-order kinetics and the results are illustrated in Fig. 7. The kapp values and corresponding R values are summarized in Table 2, indicating that plots of -ln(C/C0) vs irradiation time have good linear relationship (R2>0.98). The reaction rate constants kapp for BiOCl, BiOBr and BiOI were calculated to be 0.3949, 0.1059 and 0.0549 min-1, respectively. By comparing the rate constants estimated, the order of photocatalytic reaction rates can be placed as follows: BiOCl>BiOBr>BiOI. This result is in good agreement with the previous study of photocatalytic activity (Fig. 6).
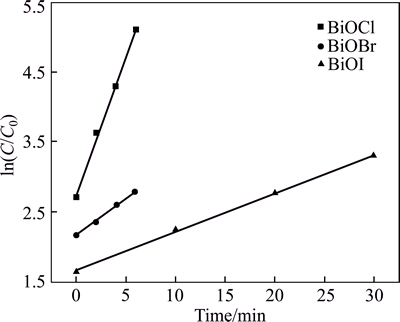
Fig. 7 Pseudo-first-order kinetics fitting plots of RhB degradation over different catalysts
The photocatalytic reaction in aqueous solution could be believed to occur on the catalyst surface, generally following the Langmuir-Hinshelwood (L-H) model as [69, 70]
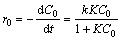 (3)
(3)
where r0 (mg/L·min), C0 (mg/L), K (L/mg), k (mg/(L·min)) and t (min) are the initial degradation rate of dye, the initial concentration of dye, the adsorption coefficient of dye, the L-H rate constant and the irradiation time, respectively. Equation (3) can be further converted into a linear form as
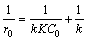 (4)
(4)
In order to demonstrate that the RhB degradation over BiOX can be well-described by the L-H model, the photocatalytic kinetics of BiOI with different initial concentrations of RhB was researched. Figure 8(a) shows that the plots of -ln(C/C0) versus t exhibit good linear relationship. Their slopes determining reaction rate constants of RhB degradation over BiOI are 0.0668, 0.0549, 0.0461 and 0.0388 min-1, corresponding to the different initial concentrations of 10, 15, 20 and 25 mg/L. Based on Eq. (4), we researched the relationship between the degradation rate of RhB and its initial concentration. As shown in Fig. 8(b), there is a good linearity (R2>0.98) between 1/r0 and 1/C0 and the fitted equation is
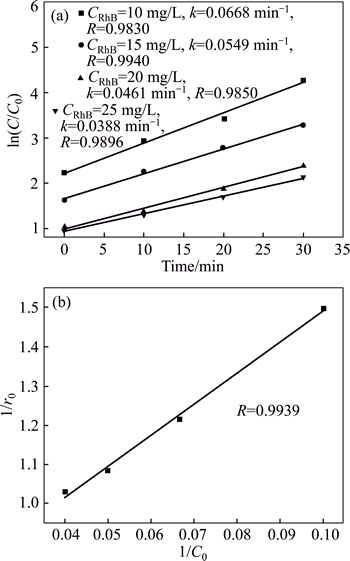
Fig. 8 Relationships of ln C0/C and reaction time with different initial concentrations of RhB (a) and 1/r0 and C0 (b)
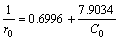 (5)
(5)
From Eq. (5), we can get the values of k and K. Then the L-H equation can be expressed as
 (6)
(6)
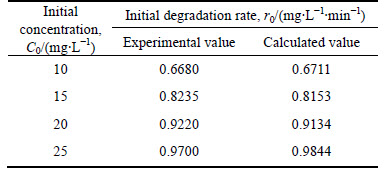
The experimental values and L-H calculated values of initial degradation rates are compared in Table 3. It can be found that the deviations between calculated values and experimental values of initial degradation rates are very small and all of the relative errors are less than 2%, indicating that RhB degradation in the presence of BiOI powers accords well with the L-H model.
Table 3 Initial velocity of photocatalytic reaction with different initial concentrations
4 Conclusions
The self-assembled hierarchical microflowers of BiOX are synthesized through general solvothermal processes. All of the as-prepared BiOX samples are pure phases and exhibit enhanced photocatalytic activity under UV-vis light irradiation. The RhB solutions could be almost degraded completely in 6 min irradiation with the existence of BiOCl and BiOBr, while it needs almost 30 min irradiation in presence of BiOI. Further study demonstrates that the relatively high photocatalytic activity of BiOCl is correlated with the relatively large specific surface area which could expose more active sites and reflect higher adsorption capacity. The photocatalytic kinetics study indicates that RhB degradation using BiOI microflowers well follows the L-H model and fits apparent first-order kinetics.
References
[1] KUBACKA A,  G. Advanced nanoarchitectures for solar photocatalytic applications [J]. Chemical Reviews, 2012, 112(3): 1555-1614.
G. Advanced nanoarchitectures for solar photocatalytic applications [J]. Chemical Reviews, 2012, 112(3): 1555-1614.
[2] ASAHI R, MORIKAWA T, IRIE H, OHWAKI T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects [J]. Chemical Reviews, 2014, 114(19): 9824-9852.
[3] OSTERLOH F E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting [J]. Chemical Society Reviews, 2013, 44(23): 2294-2320.
[4] KUDO A, MISEKI Y. Heterogeneous photocatalyst materials for water splitting [J]. Chemical Society Reviews, 2009, 38(38): 253-278.
[5] ASAHI R, MORIKAWA T, OHWAKI T, AOKI K, TAGA Y. Visible-light photocatalysis in nitrogen-doped titanium oxides [J]. Science, 2001, 293(5528): 269-271.
[6] ROY S C, VARGHESE O K, PAULOSE M, GRIMES C A. Toward solar fuels: Photocatalytic conversion of carbon dioxide to hydrocarbons [J]. ACS Nano, 2010, 4(3): 1259-1278.
[7] TONG Hua, OUYANG Shu-xin, BI Ying-pu, UMEZAWA N, OSHIKIRI M, YE Jin-hua. Nano-hotocatalytic materials: Possibilities and challenges [J]. Advanced Materials, 2012, 24(2): 229-251.
[8] CHENG Ning-yan, TIAN Jing-qi, LIU Qian, GE Chen-jiao, QUSTI A H, ASIRI A M, AL-YOUBI A O, SUN Xu-ping. Au-nanoparticle- loaded graphitic carbon nitride nanosheets: Green photocatalytic synthesis and application toward the degradation of organic pollutants [J]. ACS Appl Mater Interfaces, 2013, 5(15): 6815-6819.
[9] XIANG Quan-jun, YU Jia-guo, JARONIEC M. Graphene-based semiconductor photocatalysts [J]. Chemical Society Reviews, 2012, 41(2): 782-796.
[10] FUJISHIMA A, HONDA K. Photolysis-decomposition of water at the surface of an irradiated semiconductor [J]. Nature, 1972, 238(5358): 37-38.
[11] KHAN S U M, MOFAREH A S, INGLER W B. Efficient photochemical water splitting by a chemically modified n-TiO2 [J]. Science, 2002, 297(2): 2243-2245.
[12] KUMAR S G, DEVI L G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics [J]. The Journal of Physical Chemistry A, 2011, 115(46): 13211-13241.
[13] LINSEBIGLER A L, LU Guang-quan, YATES JR J T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results [J]. Chemical Reviews, 1995, 95(3): 735-758.
[14] CHEN C C, LU C S, CHUNG Y C, JAN J L. UV light induced photodegradation of malachite green on TiO2 nanoparticles [J]. Journal of Hazardous Materials, 2007, 141(3): 520-528.
[15] PAN Lun, ZOU Ji-jun, ZHANG Xiang-wen, WANG Li. Water-mediated promotion of dye sensitization of TiO2 under visible light [J]. Journal of the American Chemical Society, 2011, 133(26): 10000-10002.
[16] ZHANG Ke-lei, LIU Cun-ming, HUANG Fu-qiang, ZHENG Chong, WANG Wen-deng. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst [J]. Applied Catalysis B: Environmental, 2006, 68(3, 4): 125-129.
[17] LIN Xin-ping, HUANG Tao, HUANG Fu-qiang, WANG Wen-deng, SHI Jian-lin. Photocatalytic activity of a Bi-based oxychloride Bi4NbO8Cl [J]. Journal Materials Chemistry, 2007, 17(20): 2145-2150.
[18] YE Li-qun, SU Yu-rong, JIN Xiao-li, XIE Hai-quan, ZHANG Can. Recent advances in BiOX (X=Cl, Br and I) photocatalysts: Synthesis, modification, facet effects and mechanisms [J]. Environmental Science: Nano, 2014, 1: 90-112.
[19] CHENG He-feng, HUANG Bai-biao, DAI Ying. Engineering BiOX (X=Cl, Br, I) nanostructures for highly efficient photocatalytic applications [J]. Nanoscale, 2014, 6(4): 2009-2026.
[20] XIA Jie-xiang, YIN Sheng, LI Hua-ming, XU Hui, XU Li, XU Yuan-guo. Improved visible light photocatalytic activity of sphere-like BiOBr hollow and porous structures synthesized via a reactable ionic liquid [J]. Dalton Transactions, 2011, 40(19): 5249-5258.
[21] XIONG Jin-yan, CHENG Gang, LI Guang-fang, QIN Fan, CHEN Rong. Well-crystallized square-like 2D BiOCl nanoplates: Mannitol- assisted hydrothermal synthesis and improved visible-light-driven photocatalytic performance [J]. RSC Advances, 2011, 1(8): 1542-1553.
[22] ZHANG Xi, AI Zhi-hui, JIA Fa-long, ZHANG Li-zhi. Generalized one-pot synthesis, characterization, and photocatalytic activity of hierarchical BiOX (X=Cl, Br, I) nanoplate microspheres [J]. The Journal of Physical Chemistry C, 2008, 112(3): 747-753.
[23] LIU Yuan-yuan, SON W J, LU Ji-bao, HUANG Bai-biao, DAI Ying, WHANGBO M H. Composition dependence of the photocatalytic activities of BiOCl1-xBrx solid solutions under visible light [J]. Journal of Chemistry A European, 2011, 17(34): 9342-9349.
[24] HUANG Wen-lai, ZHU Qing-shan. DFT calculations on the electronic structures of BiOX (X=F, Cl, Br, I) photocatalysts with and without semicore Bi 5d states [J]. Journal of computational chemistry, 2009, 30(2): 183-190.
[25] BURDA C, CHEN Xiao-bo, NARAYANAN R, EL-SAYED M A. Chemistry and properties of nanocrystals of different shapes [J]. Chemical Reviews, 2005, 105(4): 1025-1102.
[26] ZHANG Kun, LIANG Jie, WANG Shan, LIU Jie, REN Kuai-xia, ZHENG Xiao, LUO Hui, PENG Ying-jie, ZOU Xing, BO Xu. BiOCl sub-microcrystals induced by citric acid and their high photocatalytic activities [J]. Crystal Growth Design, 2012, 12(2): 793-803.
[27] CUI Yi, LIEBER C M. Functional nanoscale electronic devices assembled using silicon nanowire building blocks [J]. Science, 2001, 291(5505): 851-853.
[28] WANG Xun, ZHUANG Jing, PENG Qing, LI Ya-dong. A general strategy for nanocrystal synthesis [J]. Nature, 2005, 437(7055): 121-124.
[29] WANG Chang-hua, SHAO Chang-lu, LIU Yi-chun, ZHANG Li-na. Photocatalytic properties BiOCl and Bi2O3 nanofibers prepared by electrospinning [J]. Scripta Materialia, 2008, 59(3): 332-335.
[30] YE Li-qun, ZAN Ling, TIAN Li-hong, PENG Tian-you, ZHANG Jiu-jun. The {001} facets-dependent high photoactivity of BiOCl nanosheets [J]. Chem Commun, 2011, 47(24): 6951-6953.
[31] HENLE J, SIMON P, FRENZEL A, SCHOLZ S, KASKEL S. Nanosized BiOX (X=Cl, Br, I) particles synthesized in reverse microemulsions [J]. Chemistry of Materials,2007, 19(3): 366-373.
[32] DENG Hong, WANG Jun-wei, PENG Qing, WANG Xun, LI Ya-dong. Controlled hydrothermal synthesis of bismuth oxyhalide nanobelts and nanotubes [J]. Chemistry-A European Journal, 2005, 11(22): 6519-6524.
[33] SONG Ji-ming, MAO Chang-jie, NIU He-lin, SHEN Yu-hua, ZHANG Sheng-yi. Hierarchical structured bismuth oxychlorides: Self-assembly from nanoplates to nanoflowers via a solvothermal route and their photocatalytic properties [J]. CrystEngComm, 2010, 12(11): 3875-3881.
[34] DENG Zheng-tao, CHEN Dong, PENG Bo, TANG Fang-qiong. From bulk metal Bi to two-dimensional well-crystallized BiOX (X= Cl, Br) micro-and nanostructures: synthesis and characterization [J]. Crystal Growth and Design, 2008, 8(8): 2995-3003.
[35] SU Jun-lin, XIAO Yang, REN Mao. Direct hydrolysis synthesis of BiOI flowerlike hierarchical structures and its photocatalytic activity under simulated sunlight irradiation [J]. Catalysis Communications, 2014, 45: 30-33.
[36] XUE Chao, XIA Jia-le, WANG Ting, ZHAO Shi-shun, YANG Gui-dong, YANG Bo-lun, DAI Yan-zhu, YANG Guang. A facile and efficient solvothermal fabrication of three-dimensionally hierarchical BiOBr microspheres with exceptional photocatalytic activity [J]. Materials Letters, 2014, 133: 274-277.
[37] PENG Sheng-jie, LI Lin-lin, ZHU Pei-ning, WU Yong-zhi, SRINIVASAN M, MHAISALKAR S G, RAMAKRISHNA S, YAN Qing-yu. Controlled synthesis of BiOCl hierarchical self-assemblies with highly efficient photocatalytic properties [J]. Chemistry-An Asian Journal, 2013, 8(1): 258-268.
[38] ZHANG Lei, CAO Xiao-feng, CHEN Xue-tai, XUE Zi-ling. BiOBr hierarchical microspheres: Microwave-assisted solvothermal synthesis, strong adsorption and excellent photocatalytic properties [J]. Journal of Colloid and Interface Science, 2011, 354(11): 630- 636.
[39] AI Zhi-hui, HO W, LEE S, ZHANG Li-zhi. Efficient photocatalytic removal of NO in indoor air with hierarchical bismuth oxybromide nanoplate microspheres under visible light [J]. Environmental Science Technology, 2009, 43(11): 4143-4150.
[40] ZHU Lu-ping, LIAO Gui-hong, BING Nai-ci, WANG Ling-ling, YANG Yang, XIE Hong-yong. Self-assembled 3D BiOCl hierarchitectures: Tunable synthesis and characterization [J]. CrystEngComm, 2010, 12(11): 3791-3796.
[41] HU Jun, WENG Sun-xian, ZHENG Zu-yang, PEI Zeng-xia, HUANG Mian-li, LIU Ping. Solvents mediated-synthesis of BiOI photocatalysts with tunable morphologies and their visible-light driven photocatalytic performances in removing of arsenic from water [J]. Journal of Hazardous Materials , 2014, 264(2): 293-302.
[42] LI Guang-fang, QIN Fan, WANG Run-ming, XIAO Sheng-qiang, SUN Hong-zhe, CHEN Rong. BiOX (X=Cl, Br, I) nanostructures: Mannitol-mediated microwave synthesis, visible light photocatalytic performance, and Cr(VI) removal capacity [J]. Journal of Colloid and Interface Science, 2013, 409(11): 43-51.
[43] CHEN Lang, HUANG Rui, XIONG Miao, YUAN Qing, HE Jie, JIA Jing, YAO Meng-yuan, LUO Sheng-lian, AU Chak-tong, YIN Shuang-feng. Room-temperature synthesis of flower-like BiOX (X=Cl, Br, I) hierarchical structures and their visible-light photocatalytic activity [J]. Inorganic Chemistry, 2013, 52(19): 11118-11125.
[44] NGUYEN T, OLLIS D F. Complete heterogeneously photocatalyzed transformation of 1, 1-and 1, 2-Dibromoethane to CO2 and HBr [J]. The Journal of Physical Chemistry, 1984, 88(16): 3386-3388.
[45] SUBRAMANIAN V, KAMAT P V, WOLF E E. Mass-transfer and kinetic studies during the photocatalytic degradation of an azo dye on optically transparent electrode thin film [J]. Industrial & Engineering Chemistry Research, 2003, 42(10): 2131-2138.
[46] OLLIS D F. Kinetics of liquid phase photocatalyzed reactions: An illuminating approach [J]. The Journal of Physical Chemistry B, 2005, 109(109): 2439-2444.
[47] DASH A, SARKAR S, ADUSUMALLI V, MAHALINGAM V. Microwave synthesis, photoluminescence, and photocatalytic activity of PVA-Functionalized Eu3+-doped BiOX (X=Cl, Br, I) nanoflakes [J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2014, 30(5): 1401-1409.
[48] CHEN Feng, LIU Hong-qi, BAGWASI S, SHEN Xing-xing, ZHANG Jin-long. Photocatalytic study of BiOCl for degradation of organic pollutants under UV irradiation [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2010, 215(1): 76-80.
[49] KUBELKA P. Ein beitrag zur optik der farban striche [J]. Z Tech Phys, 1931, 12: 593-603.
[50] LIU Jing-yi, LI Hai-ping, DU Na, SONG Shue, HOU Wan-guo. Synthesis, characterization, and visible-light photocatalytic activity of BiOI hierarchical flowerlike microspheres [J]. RSC Advances, 2014, 4(59): 31393-31399.
[51] XU Jian, MENG Wei, ZHANG Yuan, LI Lei, GUO Chang-sheng. Photocatalytic degradation of tetrabromobisphenol A by mesoporous BiOBr: Efficacy, products and pathway [J]. Applied Catalysis B: Environmental, 2011, 107(3): 355-362.
[52] XIAO Xin, ZHANG Wei-de. Facile synthesis of nanostructured BiOI microspheres with high visible light-induced photocatalytic activity [J]. Journal of Materials Chemistry, 2010, 20(28): 5866-5870.
[53] BUTLER M. Photoelectrolysis and physical properties of the semiconducting electrode WO2 [J]. Journal of Applied Physics, 1977, 48(5): 1914-1920.
[54] LUO Yuan-yuan, DUAN Guo-tao, LI Guang-hai. Synthesis and characterization of flower-like β-Ni(OH)2 nanoarchitectures [J]. Journal of Solid State Chemistry, 2007, 180(7): 2149-2153.
[55] SING K S W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984) [J]. Pure & Applied Chemistry, 2009, 57(11): 603-619.
[56] KRUK M, JARONIEC M. Gas adsorption characterization of ordered organic-inorganic nanocomposite materials [J]. Chemistry of Materials, 2001, 13(10): 3169-3183.
[57] ZHANG Jun, SHI Feng-jun, LIN Jing, CHEN Dong-feng, GAO Jian-ming, HUANG Zhi-xin, DING Xiao-xia, TANG Cheng-cun. Self-Assembled 3-D architectures of BiOBr as a visible light-driven photocatalyst [J]. Chemistry of Materials, 2008, 20(9): 2937-2941.
[58] LI Yuan-yuan, LIU Jin-ping, HUANG Xin-tang, LI Guang-yun. Hydrothermal synthesis of Bi2WO6 uniform hierarchical microspheres [J]. Crystal growth & design, 2007, 7(7): 1350-1355.
[59] LI Xiang-cun, ZHENG Wen-ji, HE Gao-hong, ZHAO Rui, LIU Dan. Morphology control of TiO2 nanoparticle in microemulsion and its photocatalytic property [J]. ACS Sustainable Chemistry & Engineering, 2014, 2(2): 288-295.
[60] ZHUANG Jian-dong, DAI Wen-xin, TIAN Qin-fen, LI Zhao-hui, XIE Li-yan, WANG Ji-xin, LIU Ping. Photocatalytic degradation of RhB over TiO2 bilayer films: Effect of defects and their location [J]. Langmuir, 2010, 26(12): 9686-9694.
[61] XUE Chao, WANG Ting, YANG Gui-dong, YANG Bo-lun, DING Shu-jiang. A facile strategy for the synthesis of hierarchical TiO2/CdS hollow sphere heterostructures with excellent visible light activity [J]. Journal of Materials Chemistry A, 2014, 2(21): 7674- 7679.
[62] TANG Jun-wang, ZOU Zhi-gang, YE Jin-hua. Effects of substituting Sr2+ and Ba2+ for Ca2+ on the structural properties and photocatalytic behaviors of CaIn2O4 [J]. Chemistry of Materials, 2004, 16(9): 1644-1649.
[63] LI Yong-yu, WANG Jian-she, YAO Hong-chang, DANG Li-yun, LI Zhong-jun. Efficient decomposition of organic compounds and reaction mechanism with BiOI photocatalyst under visible light irradiation [J]. Journal of Molecular Catalysis A: Chemical, 2011, 334(1): 116-122.
[64] WANG Xiao-meng, YANG Shao-gui, LI Hui, ZHAO Wei, SUN Cheng, HE Huan. High adsorption and efficient visible-light- photodegradation for cationic Rhodamine B with microspheric BiOI photocatalyst [J]. RSC Advances, 2014, 4(80): 42530-42537.
[65] XU Cheng-qun, WU Hong-hai, GU Feng-long. Efficient adsorption and photocatalytic degradation of Rhodamine Bunder visible light irradiation over BiOBr/montmorillonite composites [J]. Journal of Hazardous Materials, 2014, 275C(2): 185-192.
[66] LI Tian-bao, CHEN Gang, ZHOU Chao, SHEN Zao-yu, JIN Ren-cheng, SUN Jing-xue. New photocatalyst BiOCl/BiOI composites with highly enhanced visible light photocatalytic performances [J]. Dalton Transactions, 2011, 40(25): 6751-6758.
[67] JIANG Jing, ZHAO Kun, XIAO Xiao-yi, ZHANG Li-zhi. Synthesis and facet-dependent photoreactivity of BiOCl single-crystalline nanosheets [J]. Journal of American Chemistry Society, 2012, 134(10): 4473-4476.
[68] SOLTANI T, ENTEZARI M H. Sono-synthesis of bismuth ferrite nanoparticles with high photocatalytic activity in degradation of Rhodamine B under solar light irradiation [J]. Chemical Engineering Journal, 2013, 223(5): 145-154.
[69] NATARAJAN T S, NATARAJAN K, BAJAJ H C, TAYADE R J. Energy efficient UV-LED source and TiO2 nanotube array-based reactor for photocatalytic application [J]. Industrial & Engineering Chemistry Research, 2011, 50(13): 7753-7762.
[70] SONG Li-min, ZHANG Shu-jian, WEI Qing-wu. Porous BiOI Sonocatalysts: Hydrothermal synthesis, characterization, sonocatalytic, and kinetic properties [J]. Industrial & Engineering Chemistry Research, 2012, 51(3): 1193-1197.
(Edited by FANG Jing-hua)
Cite this article as:
GU Ying-ying, ZHAO Li, YANG Ming-yang, XIONG Yi-qiu, WU Zhe, ZHOU Min-jia, YAN Jun. Preparation and characterization of highly photocatalytic active hierarchical BiOX(X=Cl, Br, I) microflowers for rhodamine B degradation with kinetic modelling studies [J]. Journal of Central South University, 2017, 24(4): 754-765.
DOI:https://dx.doi.org/10.1007/s11771-017-3477-xFoundation item: Project(21301194) supported by the National Natural Science Foundation of China; Project(20130162120031) supported by Research Fund for the Doctoral Program of Higher Education of China
Received date: 2016-01-09; Accepted date: 2016-05-04
Corresponding author: YAN Jun, Professor, PhD; Tel: +86-731-88879616; Fax: +86-731-88879616; E-mail: yanjun@csu.edu.cn; guyy02@163.com
Abstract: The hierarchical BiOX (X=Cl, Br, I) microflowers were successfully synthesized via simple precipitation method at 160 °C for 24 h and characterized by XRD, SEM, TEM, UV-vis DRS and N2 adsorption-desorption techniques. The as-prepared samples were pure phases and of microflowers composed of nanosheets which intercrossed with each other. The specific surface areas were about 22.9, 17.3 and 16.2 m2/g for BiOCl, BiOBr and BiOI, respectively. The photocatalytic activities of BiOX powers were evaluated by RhB degradation under UV-vis light irradiation in the order of BiOCl > BiOBr > BiOI. Also, the kinetics of RhB degradation over BiOI was selectively investigated, demonstrating that the kinetics of RhB degradation follows apparent first-order kinetics and fits the Langmuir-Hinshelwood model.


