
Removal of SO42-, uranium and other heavy metal ions from simulated solution by sulfate reducing bacteria
WANG Qing-liang(王清良)1, 2, DING De-xin(丁德馨)2, HU E-ming(胡鄂明)2,
YU RUN-lan(余润兰)1, QIU Guan-zhou(邱冠周)1
1. School of Minerals Processing and Bioengineering, Central South University,
Changsha 410083, China;
2. Key Discipline Laboratory of National Defence for Biotechnology in Uranium Mining and Hydrometallurgy,
University of South China, Hengyang 421001, China
Received 20 September 2008; accepted 5 November 2008
Abstract:
In the case of in-situ leaching of uranium, the primitive geochemical environment for groundwater is changed since leachant is injected into the water bearing uranium deposit. This increases the concentration of SO42-, uranium and other heavy metal ions and results in the groundwater contamination. The effects of pH values of the simulated solution on the reduction of SO42- and the removal of uranium and other heavy metal ions by sulfate reducing bacteria(SRB) were studied. The results show that, when the pH value of the simulated solution is about 8, the reduction rate of SO42- by SRB and the removal rate of uranium, Mn2+, Zn2+, Pb2+ and Fe2+ will reach their highest values. A bioremediation technique for remediation of groundwater in in-situ leaching uranium mine can be developed.
Key words:
sulfate reducing bacteria; in-situ leaching of uranium; radioactively contaminated groundwater; bioremediation;
1 Introduction
Acid in-situ leaching is a new technology combining mining processing with metallurgy. The acid solution is injected into the sand mineral rock with water, and the pregnant solution is pumped out from pregnant well. Its advantages are as follows: lower cost, friendly environment and safe condition, high recovery of resource, and so on. Since 1970, the acid in-situ leaching of uranium has been studied. Several mines of acid in-situ leaching were built by studying and exploring for more than 30 years.
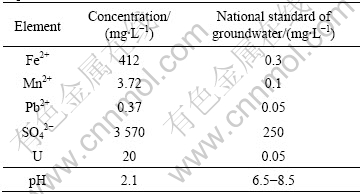
H2SO4 are used as leachant in the acid in-situ leaching of uranium in China. The geochemistry environment is changed by the injection of H2SO4, and the concentrations of SO42-, uranium and heavy mental ions increase. The pollution of groundwater is severe. The ingredients of groundwater in a well field after being exploited by in-situ leaching in China are shown in Table 1. The data in Table 1 show that the groundwater has been polluted by SO42-,uranium and heavy metal ions. The concentrations of SO42- and heavy metal ions go beyond the national standard largely.
Table 1 Ingredients of groundwater in well field after being exploited
In fact, the pollution of acid in-situ leaching of uranium was concerned many years ago. The large cost was used to do research about the physical and chemical technology to restore the polluted groundwater. The physical and chemical technology has been put forward. The research harvest has not been used due to the expensive cost. In this condition, bioremediation, a new kind of decontamination of polluted groundwater is put forward. The reducing function of sulfate reducing bacteria(SRB) to the polluted groundwater was studied by simulated polluted groundwater in lab. The removal of U, Mn, Zn, Pb and Fe was studied too. The results show that SO42-, U, Mn, Zn, Pb and Fe in polluted groundwater can be removed in certain condition. It can provide evidence to restore polluted groundwater in uranium mine of acid in-situ leaching in the future[1-7].
2 Experimental
2.1 Materials
Medium contains sodium lactate 4.0 mol/L; yeast decoction 1.0 mol/L; vitamin C 0.1 g/L; MgSO4·7H2O 0.2 g/L; K2HPO4 0.01 g/L; NaCl 10.0 g/L; and (NH4)2Fe(SO4)2·6H2O 0.2 g/L.
The water sample containing SRB was from a sewage treatment pond of paper mill in Hengyang, Hunan Province, China. The SRB was domesticated for a week in vibrator at 35 ℃ and 5 g/L Na2SO4 was put into the water sample. Then, the domestication was continued with medium. The medium was replaced once a week. After 4 weeks of domestication, SRB for lab experiment was obtained. SRB was saved in the obturation vessel at 4 ℃. The activation was done periodically in order to keep the activity of SRB.
2.2 Methods
2.2.1 Effect of pH value of solution on SRB reducing SO42-
The pH value of seven medium samples was adjusted to 4, 5, 6, 7, 8, 9 and 10 separately with 0.1 mol/L HCl and 0.1 mol/L NaOH. Then the medium samples were sterilized under the pressure of 1.4×105 Pa in the sterilization pot for 20 min. (NH4)2FeSO4 was sterilized by ultraviolet radiation and then was put into medium samples. In the meantime, the domesticated SRB was put into medium samples by 10% (volume fraction). Oxygen was removed by blowing nitrogen for 5 min. At last, the initiative pH and concentration of medium sample were analyzed. After anaerobic cultivation in vibrator at 35 ℃ for 3 weeks, the pH and concentration of medium samples were analyzed.
2.2.2 Effect of pH value of solution on removal of U, Mn2+, Zn2+, Pb2+ and Fe2+
A certain quantity of U3O8, MnCl2, ZnSO4·7H2O, Pb(NO3)2 and FeSO4·7H2O were weighed separately and then put into volume flask to dissolve. The concentrations of U, Mn2+, Zn2+ and Pb2+ were kept in 1-10 mg/L and the concentrations of Fe2+ is kept in 10-100 mg/L. The pH value of three medium samples were adjusted to 6, 7 and 8 separately with 0.1 mol/L HCl and 0.1 mol/L NaOH. Then the medium samples were sterilized under the pressure of 1.4×105 Pa in the sterilization pot for 20 min and then cooled. (NH4)2FeSO4 was sterilized by ultraviolet radiation and then was put into medium samples. In the meantime, the domesticated SRB was put into medium samples by 10% (volume fraction). Oxygen was removed by blowing nitrogen for 5 min. At last, the initiative pH and concentration of medium sample were analyzed. After anaerobic cultivation in vibrator at 35 ℃ for 3 weeks, the pH and concentration of medium sample were analyzed.
3 Results and discussion
3.1 Influence of pH value on reduction ability of SRB to sulfate
After anaerobic cultivation in vibrator at 35 ℃ for 3 weeks, the pH and concentration of medium sample were analyzed. The test results are shown in Table 2 and Figs.1 and 2.
Table 2 Effect of pH value of solution on reducibility of SO42- by SRB


Fig.1 Variation of pH value of samples during anaerobic cultivation
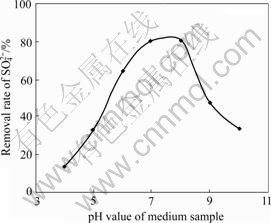
Fig.2 Effect of pH value on removal rate of SO42-
The results show that the pH value is close to 7 during anaerobic cultivation. And the reducibility of SRB to sulfate is considerably affected by the pH value. The reduction rate of SRB to sulfate is high when the pH value is 7-8.
3.2 Removal of heavy metal ions at different pH values
The concentrations of U, Mn2+, Zn2+, Pb2+, Fe2+ in solution were measured after anaerobic cultivation for 3 weeks. The results are shown in Tables 3-5.
Table 3 Removal rate of metal ions at pH 6

Table 4 Removal rate of metal ions at pH 7

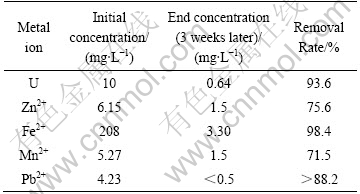
Table 5 Removal rate of metal ions at pH 8
The results in Table 3 show that the decontamination effects of U, Fe2+ and Pb2+ are quite good and the decontamination effects of Zn and Mn2+ are unsatisfied.
The results in Table 4 show that the decontamination effects of all the metal ions are good, and the decontamination effects of Zn2+ and Fe2+ are the best.
The results in Table 5 show that the removal rates are high for all the metals at pH 8 and the decontamination effects of U, Fe2+ and Pb2+ are the best.
4 Conclusions
1) The influence of pH on the reducing ability is considerable. The reducing rate is high when pH values are between 6 and 8. The reducing effect is the best when the pH value is 8, and after three weeks, the reducing rate of SO42- is up to 80.17%.
2) The influence of pH value on the decontamination effects of U, Fe2+ and Pb2+ is considerable. The decontamination effects of U, Fe2+ and Pb2+ are quite good when pH value is 6, and the decontamination rates are all above 75%. But the decontamination rates of Zn2+ and Mn2+ are not good and the decontamination rates are only 16.3% and 11.6%, respectively. When the pH value is 7, the decontamination effects of all metal ions are good. When the pH value is 8, the decontamination rates of U and Fe2+ are 93.6% and 98.4%, respectively.
3) When the pH value of solution is 8, the reducing effect of SO42- and the decontamination effects of U, Mn2+, Zn2+, Pb2+, Fe2+ are both very good.
References
[1] WANG Hai-feng, TAN Ya-fei. Well field technics of uranium in-situ leaching [M]. Beijing: Metallurgical Industry Press, 2002: 11. (in Chinese)
[2] RAJESHK H, BRENTM S. Uranium immobilization by sulfate-reducing biofilms [J]. Environ Sci Technol, 2004, 38: 2067-2074.
[3] WANG Hai-feng. Technology and practice of uranium in-situ leaching [M]. Beijing: Atomic Energy Press, 1998: 4. (in Chinese)
[4] LOVLEY D R. Bacteria remove uranium from groundwater [J]. Industrial Bioprocessing, 2003, 25(12): 8-9.
[5] BARTON LY L, CHOUDHURY K. Bacterial reduction of soluble uranium: The first step of in situ immobilization of uranium [J]. Radioactive Waste Management and Environmental Restoration, 1996, 20(2/3): 141-151.
[6] BARTON L L, NUTTALL H E, et al. Bioremediation of ground water contaminants at a uranium mill tailings site [C]// Proceedings of the International Conference on Radioactive Waste Management and Environmental Remediation. ICEM, 1995: 1579-1583.
[7] YANG Jing-liang, ZHAO Yi, REN Hong-qiang, et al. Study on the effect factors of biological reduction of sulfate [J]. China Biogas, 1999, 17(2): 7-13. (in Chinese)
[8] ZHAO Yu-hua, YE Yang-fang. Sulfate reducing bacteria and influence factors [J]. Environment Pollution and Control, 1997, 19(5): 41-43. (in Chinese)
[9] MA Xiao-hang, JIA Xiao-ming, ZHAO Yu-hua. Research and application of the processes of disposal of wastewater containing heavy metals by sulfate reducing bacteria [J]. Journal of Microbiology, 2003, 1: 36-39. (in Chinese)
[10] SPEAR J R, FIGUEROA L A, HONEYMAN B D. Modeling reduction of uranium U(VI) under variable sulfate concentrations by sulfate-reducing bacteria [J]. Applied and Environmental Microbiology, 2000, 66(9): 3711-3721.
[11] YI Zheng-ji, TAN Kai-xuan, TAN Ai-li. Influence of environmental factors on reductive bioprecipitation of uranium by sulfate reducing bacteria [J]. International Biodeterioration & Biodegradation, 2007, 60(4): 258-266.
[12] BENEDETTO J S, DE ALMEIDA S K, GOMES H A. Monitoring of sulfate-reducing bacteria in acid water from uranium mines [J]. Minerals Engineering,2005, 18(13/14): 1341-1343.
[13] LEE B D, WALTON M R, MEGIO J L. Biological and chemical interactions with U(VI) during anaerobic enrichment in the presence of iron oxide coated quartz [J]. Water Research, 2005, 39(18): 4363-4374.
[14] XIE Shui-bo, YANG Jing, CHEN Chao. Study on biosorption kinetics and thermodynamics of uranium by Citrobacter freudii [J]. Journal of Environmental Radioactivity, 2008, 99: 126-133.
[15] GROUDEV S, GEORGIEV P, SPASOVA I. Bioremediation of acid mine drainage in an uranium deposit [J]. Advanced Materials Research, 2007, 20/21: 248-257.
[16] YI Zheng-ji, TAN Kai-xuan, YU Zhen-xun. Synergistic effect of combining sulfate reducing bacteria and zerovalent iron permeable reactive barriers on the treatment of groundwater rich in uranium, sulfate and heavy metals [J]. Chinese Journal of Geochemistry, 2006, 25(S1): 125-126.
[17] KONDO R, BUTANI J. Comparison of the diversity of sulfate-reducing bacterial communities in the water column and the surface sediments of a Japanese meromictic lake [J]. Limnology, 2007, 8(2): 131-141.
Foundation item: Project(10475038) supported by the National Natural Science Foundation of China
Corresponding author: WANG Qing-liang; E-mail: nhdxwql@yahoo.com
(Edited by YUAN Sai-qian)


