In situ formation of titania film on NiTi alloy treated with hydrogen peroxide solution at low temperature
CHU Cheng-lin(储成林)1, ZHOU Jun(周 俊)1,
CHUNG Jonathan-CY(钟志源)2, PU Yao-pu(浦跃朴)3, LIN Ping-hua(林萍华)1
(1. Department of Materials Science and Engineering, Southeast University, Nanjing 210096, China;
2. Department of Physics and Materials Science, City University of Hong Kong, Hong Kong, China;
3. School of Public Health, Southeast University, Nanjing 210096, China)
Abstract:
Chemically polished NiTi shape memory alloy(SMA) substrate was treated with a boiling aqueous solution containing hydrogen peroxide to form titania film in situ at low temperature. The surface characterizations of titania film on NiTi substrate were investigated by scanning electron microscopy, X-ray diffractometry and X-ray photoelectron spectroscopy. The results show that titania film is successfully fabricated in situ on NiTi SMA by this surface oxidation method. It is mainly composed of rutile and anatase, whose surface compositions and morphologies are sensitive to H2O2 content. In situ formation mechanism of titania film on NiTi substrate was discussed based on the experimental results.
Key words:
titania; thin films; shape memory alloy(SMA); NiTi alloy; oxidation; surface structure CLC number: TB43;
Document code: A
1 INTRODUCTION
NiTi shape memory alloy(SMA) has been widely used as biomedical applications because of its unique properties, such as shape memory effect and superelastic properties[1, 2]. It is well known that the biocompatibility of NiTi implants depends on a native corrosion-resistant titania film on the surface of NiTi avoiding the allergic and toxic effects of nickel[3]. However, nickel either in metallic or oxidized state is also detected on the surface of NiTi, and its amount depends on the surface treatments[4, 5]. Therefore, it is useful to develop the fabrication approaches of ideal titania film on NiTi SMA, e.g. sol-gel method[6].
Note the fact that NiTi SMA contains a large amount of Ti, which can be oxidized in the environment containing oxygen and leads to in situ formation of titania. Thus the surface oxidation method may be an ideal process to fabricate titania film for NiTi SMA. Up to now, several high-temperature oxidation methods as well as oxidation behaviors and surface characterizations of NiTi SMA have been reported[7-11]. It is found that NiTi SMAs after different surface oxidations at high temperatures are predominantly covered with titania, which suggests that in situ formation of titania film on NiTi SMAs by surface oxidation method is possible. It should be pointed out that the phase transformation behaviors in NiTi SMA are very sensitive to the heat treatment conditions, such as temperature[2]. As a result, the surface oxidation treatment at high temperature will have an undesired effect on shape memory properties of NiTi SMAs.
Many studies indicate that titania films can be successfully synthesized in situ by a low-temperature oxidation of pure Ti substrates in the solutions containing hydrogen peroxide[12-14]. However, few systematic literatures have been reported to date to investigate the in situ formation of titania films on the surfaces of NiTi SMAs at low temperature. In the present work, chemically polished NiTi substrates were treated with a boiling aqueous solution containing hydrogen peroxide to form titania film in situ at low temperature.
2 EXPERIMENTAL
A commercially available NiTi (50.8% Ni, molar fraction, the same below) SMA plate for medical application with a martensite start temperature(Ms) of -12.8℃ and an austenite finish temperature(Af) of 33.4℃ was cut into small rectangular blocks (10mm×10mm×1mm). All samples were cleaned separately in acetone, ethanol and deionized water for 10min, and then chemically polished to remove the original oxides on the surface for 10min in Krolls reagent: a mixture of 2mL hydrofluoric acid (HF, 40%), 4mL nitric acid (HNO3, 40%) and 994mL deionized water. The samples were subsequently ultrasonically washed in acetone for 10min and in deionized water for 10min. They were divided into three groups. The first group was used as control. The second group and the third one were further oxidized in the boiling aqueous solutions containing 10% H2O2 and 30% H2O2 for 2h, respectively, and then ultrasonically rinsed with deionized water for 10min.
Samples were analyzed by XPS using a VG Scientific ESCALAB 5 spectrometer with monochromatic AlKα X-ray radiation and 20eV pass energy. The high-resolution XPS spectra over the Ti2p, O1s and Ni2p ranges were used for assessment of the chemical state as well as for quantification. The phases of the films were characterized by thin-film X-ray diffraction using an X-ray diffractometer (RAD ⅡA, Rigaku, Japan) operated with CuKα under 40kV and 25mA, equipped with a thin-film attachment on which the glancing angle was 1°. The surface morphologies of the samples were observed by a Philips XL30 FEG scanning electron microscope(SEM) after the surface was coated with gold films.
3 RESULTS AND DISCUSSION
Fig.1 and Fig.2 show the SEM photographs of the surface of NiTi SMAs. There are some submicron pores on the surface of the chemically polished NiTi SMA, which may be due to the treatment with Krolls reagent. Oxide films are present on the surfaces of H2O2-oxidized NiTi substrates. The surface morphology of the films is sensitive to the concentration of H2O2 in the aqueous solutions. The oxide film on the sample treated with the 10% H2O2 solution is loose, while the 30% H2O2-oxidized NiTi SMA is covered by a homogeneously dense oxide film with some micro-cracks.
Table 1 lists the chemical compositions of the surfaces of the chemically polished NiTi SMA and the H2O2-oxidized NiTi ones, respectively. Ti, Ni and O signals are detected for all samples. The amount of oxygen on the surface of the freshly polished NiTi sample is only about 7.9%, which indicates that the original oxides on the surface of NiTi SMAs have been removed successfully. After NiTi SMA treated by H2O2 solution, O becomes the dominant surface element, whose concentration

Fig.1 SEM image of surface of chemically polished NiTi SMA
Table 1 Chemical composition of surface of NiTi SMAs(molar fraction, %)
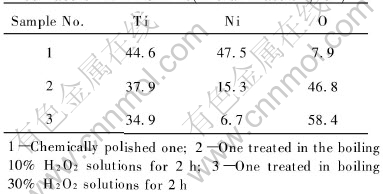
changes with the concentration of H2O2 (46.8% for the 10% H2O2-oxidized NiTi and 58.4% for the 30% H2O2-oxidized one). Moreover, the surface concentration of Ni decreases remarkably from 47.5% for the chemically polished sample to 15.3% for the 10% H2O2-oxidized one and 6.7% for the 30% H2O2-oxidized one.
Fig.3 shows the high resolution Ti2p XPS spectra of the surfaces of two H2O2-oxidized NiTi SMAs. Two dominant peaks for both samples are identified as Ti4+(TiO2)2p3/2 at 459.3eV and Ti4+(TiO2) 2p1/2 at 464.8eV. Some remnants of combined Ti species (TiNi-Ti) in the intermetallic NiTi state can also be found by the presence of the small shoulders near 454.5eV and 460.5eV on the curves, which correspond to the binding energies of TiNi-Ti2p3/2 and 2p1/2 spin states[15].
Fig.4 shows the high resolution Ni2p XPS spectra of the surfaces of NiTi SMAs treated in the boiling aqueous solutions containing different concentrations of hydrogen peroxide. They are consistent with each other, and mainly consist of two major peaks. The binding energies of two peaks are about 853.8eV and 870.5eV, which correspond to NiNi-Ti2p3/2 and 2p1/2 in the intermetallic NiTi state rather than free Ni(Ni02p3/2 of 853.0eV)[15]. The Ni oxide peak could not be found for both samples. Moreover, NiNi-Ti2p spec-tra exhibit an additional satellite structure, which
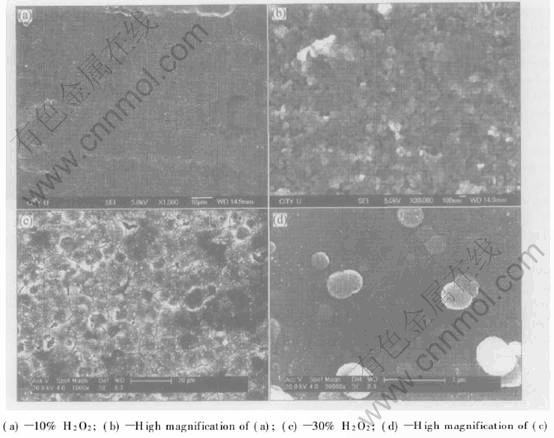
Fig.2 SEM images of surface of NiTi SMAs treated in boiling aqueous solutions containing different concentrations of hydrogen peroxide for 2h
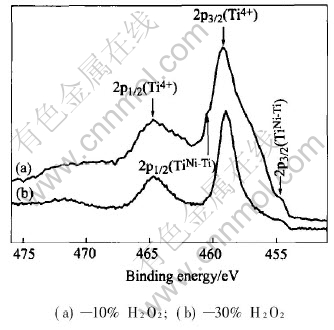
Fig.3 Ti2p XPS spectra of surface of NiTi SMAs treated in boiling aqueous solutions containing different concentrations of hydrogen peroxide for 2h
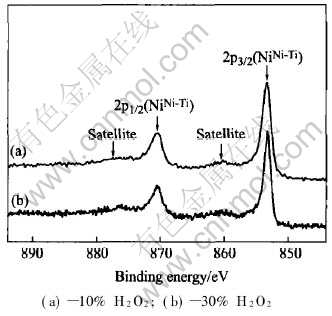
Fig.4 Ni2p XPS spectra of surface of NiTi SMAs treated in boiling aqueous solutions containing different concentrations of hydrogen peroxide for 2h
is separated from the main peaks by 7eV as shown in Fig.4. This additional satellite structure coincides with that observed in NiTi samples in the literature[4].
Fig.5 shows the XRD patterns of the surfaces of NiTi SMAs treated in the boiling aqueous solutions containing different concentrations of hydrogen peroxide. It can be found that except for the peak of the crystal plane (110) corresponding to intermetallic NiTi substrate phase, the broad peaks associated with the crystal planes (110) and (101) of rutile and the crystal plane (101) of anatase are present for both samples. Obviously the XRD patterns display a consistent result with the XPS spectra that a titania film mainly consists of poorly crystallized rutile and anatase phases formed on the H2O2-oxidized NiTi SMA. Both Ni oxide and free Ni could not be detected from XRD and XPS spectra, thus the remnant Ni in titania films must be presented as intermetallic NiTi phase, which has also been indicated by XPS analysis. The XRD peak corresponding to intermetallic NiTi phase in Fig.5 may derive from both the remnant NiTi phase in the titania film and the NiTi substrate.
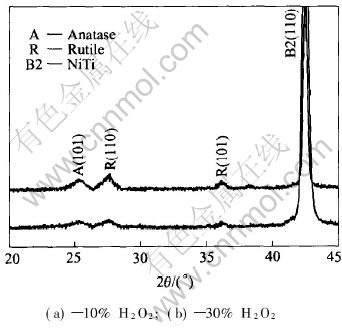
Fig.5 XRD patterns of surface of NiTi SMAs treated in boiling aqueous solutions containing different concentrations of hydrogen peroxide for 2h
In situ formation mechanism of titania films on NiTi substrates in the boiling H2O2 aqueous solution was proposed as follows based on our experimental results. As discussed in the literature[15], most metals react with oxygen at surface imperfections to form an oxide layer with a release of free energy. In this study, the oxidizing reaction of NiTi SMA proceeds by the decomposition of H2O2 into oxygen. It is estimated that the enthalpy of TiO2 formation (-956kJ/mol) is almost four times of the one for NiO formation (-241kJ/mol)[16], which may reflect the stronger affinity of Ti than that of Ni for oxygen chemisorption. Therefore, Ti on the outermost surface of NiTi is oxidized firstly by oxygen derived from the decomposition of H2O2 to form TiO2 while Ni remains unchanged. Ni atoms can be removed from the Ni-Ti bond due to H2O2-etching, left from the surface and are released into the aqueous solution during boiling, while Ti bound in oxide is more stable and not released into the aqueous solution. The release of Ni atoms from NiTi surface into the boiling water has already been found by Shabalovskaya and Anderegg with the aid of inductively coupled plasma analysis(ICPA)[4]. The release of Ni atoms leads to the formation of vacancies on NiTi substrate, which can promote the further reaction of oxygen with Ti atoms. The predominant phase TiO2 in the oxide film has a larger unit cell volume (0.062nm3 for rutile) than austenitic NiTi substrate (0.027nm3)[15]. The larger TiO2 crystal structure is accommodated by O migration towards the NiTi substrate and Ni migration towards the surface. As the oxidation progresses, O atoms diffuse inward while Ni atoms diffuse outward through the oxide film. Finally, the oxide film mainly comprised TiO2 and depleted relatively in Ni forms on NiTi substrate. Obviously in situ formation of titania film on NiTi substrates in the boiling H2O2 aqueous solution is dominated by in situ oxidation of surface component Ti and the synchronous removal of undesired Ni into the aqueous solution. The growth rate of titania film is mainly controlled by the diffusion process of O and Ni atoms in titania film. Owing to the volume difference between the oxide and NiTi substrate, high stress will be built up in titania film, especially in dense titania film during its growing process. As a result, some microcracks form in titania film on the H2O2-oxidized NiTi SMA, as shown in Fig.2.
Although the surface concentration of Ni could decrease remarkably to 6.7% after NiTi was oxidized by 30% H2O2 solution, the remnant Ni in titania film is undesired and must be removed as greatly as possible. The remnant of Ni presented as NiTi phase in titania film may be due to the insufficient oxidation reaction of NiTi substrate and the incomplete removal of Ni into the aqueous solution under the given conditions, which is associated with the relatively low reactivity ability of Ti with O and the relatively low diffusion rate of O and Ni atoms at low temperature. The formation of titania films with different surface morphologies and compositions as shown in Fig.2 may be related with different oxidation abilities of the boiling aqueous solutions containing different concentrations of H2O2. Moreover, the crystalline of titania films on two H2O2-oxidized NiTi SMAs is poor and needs to be improved. The optimized techniques to fabricate ideal titania films on NiTi substrates using this low-temperature oxidation method will be reported in the future.
4 CONCLUSIONS
Titania film is successfully fabricated in situ on NiTi SMA by surface oxidation in a boiling H2O2 aqueous solution at low temperature. Titania film is mainly composed of rutile and anatase, whose surface compositions and morphologies are sensitive to H2O2 content in the aqueous solution. In situ formation of titania film on NiTi substrate is dominated by in situ oxidation of surface component Ti and the synchronous removal of undesired Ni into the aqueous solution.
REFERENCES
[1]Duerig T, Pelton A, Stockel D. An overview of nitinol medical applications [J]. Mater Sci Eng, 1999, A273-275: 149-160.
[2]Otsuka K, Wayman C M. Shape Memory Materials [M]. Cambridge: Cambridge University Press, 1998.
[3]Wever D J, Veldhuizen A G, Vries J D E, et al. Electrochemical and surface characterization of a nickel-titanium alloy [J]. Biomaterials, 1998, 19: 761-769.
[4]Shabalovskaya S A, Anderegg J W. Surface spectroscopic characterization of TiNi nearly equiatomic shape memory alloys for implants [J]. J Vac Sci Technol, 1995, A13(5): 2624-2632.
[5]Filip P, Lausmaa J, Musialek J, et al. Structure and surface of TiNi human implants [J]. Biomaterials, 2001, 22: 2131-2138.
[6]LIU J X, YANG D Z, SHI F, et al. Sol-gel deposited TiO2 film on NiTi surgical alloy for biocompatibility improvement [J]. Thin Solid Films, 2003, 429(1-2): 225-230.
[7]Armitag D A, Grant D M. Characterisation of surface-modified nickel titanium alloys [J]. Mater Sci Eng, 2003, A349: 89-97.
[8]Firstov G S, Vitchev R G, Kumar H, et al. Surface oxidation of NiTi shape memory alloy [J]. Biomaterials, 2002, 23: 4863-4871.
[9]XU C H, MA X Q, SHI S Q, et al. Oxidation behavior of TiNi shape memory alloy at 450-750℃ [J]. Mater Sci Eng, 2004, A371: 45-50.
[10]CHU C L, WU S K, YEN Y C. Oxidation behavior of equiatomic TiNi alloy in high temperature air environment [J]. Mater Sci Eng, 1996, A216: 193-200.
[11]Chan C M, Trigwell S, Duerig T. Oxidation of a NiTi alloy [J]. Surf Interface Anal, 1990, 15: 349-354.
[12]Takemoto S, Yamamoto T, Tsuru K, et al. Platelet adhesion on titanium oxide gels: effect of surface oxidation [J]. Biomaterials, 2004, 25: 3485-3492.
[13]WU J, Hayakawa S, Tsuru K, et al. Porous titania films prepared from interactions of titanium with hydrogen peroxide solution [J]. Scripta Materialia, 2002, 46: 101-106.
[14]WANG X X, Hayakawa S, Tsuru K, et al. Improvement of bioactivity of H2O2/TaCl5-treated titanium after subsequent heat treatments [J]. J Biomed Mater Res, 2000, 52: 171-176.
[15]Green S M, Grant D M, Wood J V. XPS characterization of surface modified Ni-Ti shape memory alloy [J]. Mater Sci Eng, 1997, A224: 21-26.
[16]Weast R C, Astle M J. CRC Handbook of Chemistry and Physics [M]. Florida: CRC Press, 1982.
Foundation item: Project(CityU 1181/01E) supported by the Research Grants Council of the Hong Kong Special Administrative Region, China; Project(BK2003062) supported by the Natural Science Foundation of Jiangsu Province, China
Received date: 2004-11-29; Accepted date: 2005-03-21
Correspondence: CHU Cheng-lin, PhD; Tel: +86-25-83996387; E-mail: clchu@seu.edu.cn


