
Influence of Si on stability of TiC in Al melts
DING Hai-min1, 2, LIU Xiang-fa2
1. Department of Mechanical Engineering, North China Electric Power University, Baoding 071003, China;
2. Key Laboratory for Liquid-Solid Structural Evolution and Processing of Materials of Ministry of Education,
Shandong University, Ji’nan 250061, China
Received 27 August 2010; accepted 20 December 2010
Abstract:
The influence of Si on the stability of TiC in Al melts was studied. It is found that TiC particles in Al melts become unstable with the addition of Si. When the melting temperature is below 890 °C, TiC will react with Al and Si to form TiAlxSiy and Al4C3 phases. But if the melt temperature is above 890 °C, TiC will react with Al and Si to form to Ti3SiC2 and Al4C3. It is considered that the influence of Si on the stability of TiC in Al melts is due to its incursion into TiC crystal lattice during the holding, which will cause serious lattice distortion in TiC and then speed up the out-diffusion of surrounded C atoms.
Key words:
TiC; silicon; Al-Si alloy; stability;
1 Introduction
Metal matrix composites (MMCs) are studied in numerous research work due to their good physical and mechanical properties. For Al based MMCs, SiC, Al2O3, TiC and TiB2 are the most widely used reinforcement phases. It has been reported that, among them, TiC is particularly attractive in terms of its high hardness and elastic modulus, low density and good wettability with molten aluminum [1-2]. In addition, TiC can also be used as the reinforcement phase for Mg [3] and Cu alloys [4-5]. Furthermore, TiC particles are efficient substrates for the nucleation of α(Al). The Al-Ti-C master alloys are considered the perfect substitutes for the Al-Ti-B master alloys in the refinement of Al alloys [6-7].
It is known that, whether as the reinforcement phase or nucleation, TiC must be stable. But it was reported that TiC can easily react with other elements in Al melts and the instability of TiC will inevitably influence its application in the composites or refinement. For example, KENNEDY et al [8] found that TiC particles in TiC reinforced Al based composites can react with Al at the interface to form a brittle Al4C3 phase which will degrade the properties of the composites. In addition, many researchers found that the refining efficiency of Al-Ti-C master alloys will be faded when Al melts are held for more than 15 min. Most of researchers attributed the fading of the refinement efficiency to the instability of TiC in Al melts [9-10]. So controlling the instability of TiC is an important aspect for its application.
Furthermore, it was noticed that when a certain amount of Si exists in Al melts, the fading of the refinement efficiency of the Al-Ti-C master alloys will much faster and more serious [11-12]. This indicates that Si can remarkably influence the stability of TiC. It is known that Al-Si alloys are widely used in the industry and most of them need to be refined before application. But due to the influence of Si on TiC, the application of TiC in Al-Si alloys must be prudent.
So, in the present work, the influence of Si on the stability of TiC in Al melts was systematically studied in order to clarify the possibility and conditions of application of TiC in Al-Si alloys. In addition, the mechanism of the influence of Si on TiC was also discussed.
2 Experimental
The Al-3Ti-0.75C master alloy was firstly prepared by a melt reaction method. 99.5% pure Ti powder, 99.8% pure graphite with the size of 10 ?m and 98.0% pure Al powder along with 99.7% commercial pure Al were used to produce Al-Ti-C master alloy. Ti and Al powders along with the graphite were firstly mixed and ball milled for 10 h, and then the ball milled mixtures were cold-pressed into columnar compacts. After that, the compacts were added into the commercial pure Al melt at above 1 000 °C. After holding for about 10 min, the melt was poured into a mold. The obtained Al-3Ti-0.75C master alloy was designated as Sample-1.
Then the Al-3Ti-0.75C master alloy was re-melted to 750 °C in a 5 kW-electric resistance furnace and 7% and 13% (mass fraction) Si were added into the melt, respectively. After holding for 10 min, the melt was poured into an iron chill mould with dimensions of 75 mm×35 mm×20 mm. The sample added with 7% Si was designated as Sample-2 and the other added with 13% Si was designated as Sample-3. In addition, the influence of holding temperatures on the evolvement of TiC was examined.
The Al-Ti-C and Al-Ti-C-Si samples were analyzed with X-ray diffractometer using Cu Kα radiation, in 2θ range of 20° and 100° at a step rate of 4 (°)/min. Metallographic samples were mechanically ground and polished through standard routines. The microstructures of them were analyzed by electron probe microanalysis (EPMA). Furthermore, the prepared Al-Ti-C alloy was processed into powder and then was mixed with about 7% and 13% (%) Si powders, respectively. Then the mixtures of the powders were cold-pressed into disc compacts. A Netzsch 404 differential scanning calorimeter (DSC) was used to analyze these compacts. The Al-Ti-C-7Si alloy (Sample-2) was also analyzed by DSC. All the samples were heated in alumina crucibles at a heating rate of 10 K/min to 1 100 °C under flowing argon. After reaching the maximum temperature, the furnace was switched off and allowed to cool naturally to room temperature.
3 Results and discussion
3.1 Microstructure of Al-Ti-C master alloy
Figure 1(a) shows the microstructure of the Al-3Ti-0.75C master alloy (Sample-1). It can be seen that a lot of bright particles are uniformly distributed in Al matrix. These particles were then extracted using hydrochloric acid from the alloy and analyzed with TEM. The results shown in Figs. 1(b) and 1(c) confirm that the particles are TiC. Figure 1(d) shows the XRD pattern of Sample-1. The reflections of TiC can be observed apart from α(Al), and no other phases exist in the result, further indicating the successful preparation of the Al-Ti-C master alloy.
3.2 Influence of Si content
Figure 2 shows the microstructures of the obtained Al-Ti-C-7Si alloy (Sample-2) and Al-Ti-C-13Si alloy (Sample-3). The holding temperature was 750 °C and the holding time was 10 min. From Fig. 2(a), it is found that the microstructure of Sample-2 is very different from that of the Sample-1. Most of TiC particles disappeared and transformed to a bright phase along with a black block-like phase. Most of the bright phase is block-like and some is needle-like. It is found from Fig. 3 that the bright phase mainly contains Al, Ti and Si elements, indicating that it is a kind of TiAlxSiy ternary phase.

Fig. 1 Microstructures and XRD pattern of Al-3Ti-0.75C master alloy (Sample-1): (a) Microstructure of Sample-1; (b) Morphology of particles; (c) Corresponding selected area diffraction (SAD) pattern of a particle in (b); (d) XRD pattern of Sample-1

Fig. 2 Microstructures of Sample-2 (a) and Sample-3 (b)

Fig. 3 EDS analysis for bright blocky phase in Sample-2: (a) Microstructure of Sample-2; (b) EDS result of Point A in (a)
The map-analysis results of Sample-2 are present in Fig. 4. The results further confirm that the bright phase is the TiAlxSiy ternary phase. In addition, it is noted that the black blocky phase mainly contains Al, C and O. It is deduced that this phase should be Al4C3 phase, and O element is brought in by reaction between Al4C3 and H2O during polishing the samples.
In order to further make sure the constituents of the phases, the microstructure of Sample-2 was line- analyzed by EPMA, as shown in Fig. 5. It is noted that the bright blocky phase mainly contains Ti, Si and Al, while the black phase mainly contains C, Al and O. This further confirms that they are ternary TiAlxSiy and Al4C3 phases.
In addition, from Figs. 4 and 5, it can be seen that some TiC particles are residual in Sample-2. Most of residual TiC particles don not disperse uniformly in the Al matrix, but agglomerate around TiAlxSiy or Al4C3 phases. It should be noticed that some residual TiC particles not only contain Ti and C but also have some Si element. One of these TiC is marked in Fig. 5(b) by line ①. This indicates that Si has penetrated into TiC particles to form Si-containing TiC particles before they transformed to TiAlxSiy and Al4C3. These Si-containing TiC should be the transitional phase between TiC and TiAlxSiy/Al4C3.
In Figure 2(b), the bright block-like phase and black blocky Al4C3 phase are observed. The bright block-like phase which is bigger than that in Sample-2 also mainly contains Ti, Al and Si elements. In addition, some TiC particles still exist in Sample-3 and form aggregations with other phases. Figure 6 shows the line-analysis results of these aggregations. It is confirmed that the small bright particles are TiC. One of them is marked by line ② in Fig. 6(b) and it is obviously found that, like the TiC shown in Fig. 5, some residual TiC particles also contain Si element.
Figure 7 shows XRD patterns of Sample-1, Sample-2 and Sample-3. Compared with the XRD pattern of the Al-3Ti-0.75C alloy, it is further confirmed that most of TiC particles have transformed to TiAlxSiy and Al4C3 after the addition of Si.
3.3 Influence of melting temperature
The melting temperature is an important factor for the transformation of TiC in Al-Si alloys. Figure 8 shows microstructures of Al-Ti-C-7Si samples poured at 750, 800, 900 and 1 000 °C, respectively. It is found that TiC particles reacted with Al and Si to form TiAlxSiy and Al4C3 phases at 750 and 800 °C. But when the holding temperatures were 900 °C and 1 000 °C, most of the blocky TiAlxSiy phases disappeared and new phases occurred. Some of these phases are short needle-like and some are particle-like, and the size of them ranges from 2 μm to 7 μm.
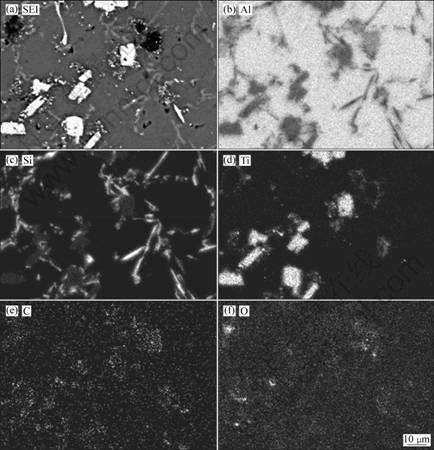
Fig. 4 EPMA mapping analysis of Sample-2: (a) SEI image; (b)-(f) Element mapping of Al, Si, Ti, C and O
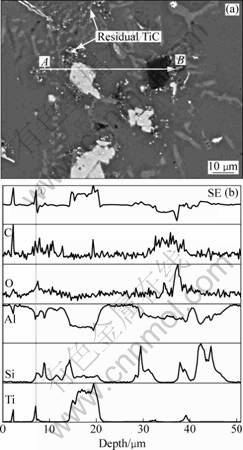
Fig. 5 EPMA line scanning analyses of sample-2 from A to B: (a) Microstructure; (b) Elements distribution
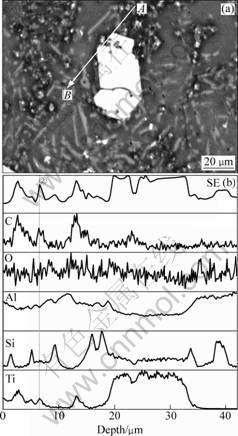
Fig. 6 EPMA line scanning analyses of sample-3 from A to B: (a) Microstructure; (b) Elements distribution
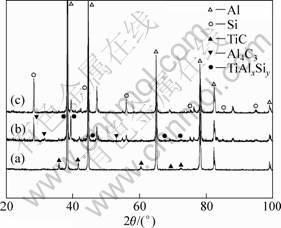
Fig. 7 XRD patterns of Sample-1 (a), Sample-2 (b) and Sample-3 (c)
The results of EPMA line-analysis are shown in Fig. 9. It found that the new formed phases mainly contain Ti, C and Si elements (marked by line ③ and ④ in Fig. 9). The XRD results in Fig. 10 indicate that TiAlxSiy and Al4C3 phases obviously decrease when the holding temperatures of melt are 900 and 1 000 °C and the reflection of Ti3SiC2 appears. This confirms that the new formed phases in Fig. 9 are Ti3SiC2.
3.4 Thermal analysis of reaction
The DSC analysis result of the mixture of Al-Ti-C alloy with 7% Si powder is shown in Fig. 11(a). It can be seen that the melting endothermic event begins at about 570 °C and ends at about 660 °C. After that, at approximately 897 °C, another endothermic event is observed. There is only a small exothermic peak between 660 and 897 °C. The beginning of this exothermic event is at about 775 °C. The DSC analysis result of Al-Ti-C with about 13% Si powder is shown in Fig. 11(b). The result is very similar to Fig. 11(a). Besides the melting peak, there is another obvious endothermic event at about 895 °C. A small exothermic peak is observed at about 778 °C. In addition, the Al-Ti-C-7Si alloy (Sample-2) is also analyzed by DSC. The result is shown in Fig. 11(c). Compared with Figs.11(a) and (b), there are only two obvious endothermic events and no any exothermic event is observed.
According to the study of WANG et al [13], reaction (1) is an endothermic event which happens at about 881 °C.
![]() (1)
(1)
Based on the evolvement of TiC mentioned above, the endothermic event which begins at about 897 °C in Fig. 11(a) and 895 °C in Fig. 11(b) should be due to the reaction (2). So before that, there must be a reaction (3) to form the TiAlxSiy and Al4C3 phases. But only a small exothermic event is found at about 775 °C. It is deduced that reaction (3) is a gradual process in the melt. After the alloy is melted, the reaction can begin and the reaction rate will get the highest between 760 and 790 °C.
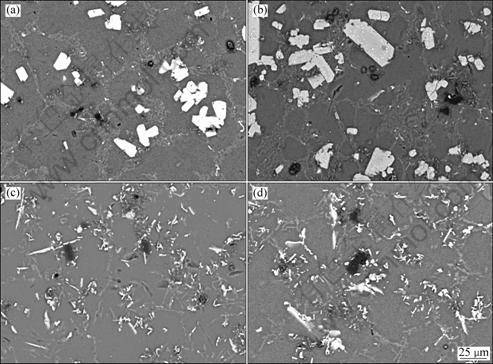
Fig. 8 Microstructures of Al-Ti-C-7Si alloys held at different temperatures: (a) 750 °C; (b) 800 °C; (c) 900 °C; (d) 1 000 °C
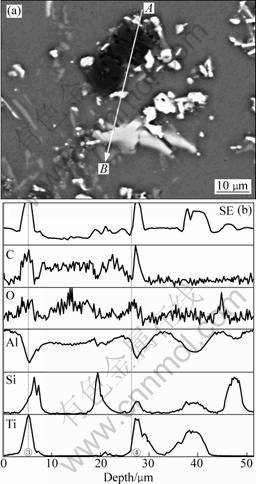
Fig. 9 Line scanning analyses from A to B of Al-Ti-C-7Si alloy held at 1 000 °C: (a) Microstructure; (b) Element distribution

Fig. 10 XRD patterns of Al-Ti-C-7Si alloy held at 800 °C (a), 900 °C (b) and 1 000 °C (c)
![]() (2)
(2)
![]() (3)
(3)
For Fig. 11(c), most of the TiC particles in the Sample-2 have transformed to TiAlxSiy and Al4C3 before the DSC experiment, so in the DSC traces, there is no any exothermic event.
According to the study of KENNEDY et al [8], the reaction between Al and TiC in Al-TiC MMCs can occur at 600 °C, the highest reaction rate is observed between 700 °C and 750 °C; above 900 °C, this reaction is no longer observed. When there is Si element in the melt, the reaction process among TiC, Al and Si is almost the same with the reaction between TiC and Al. The thermodynamics conditions of the reactions are not changed very much. The main work of Si is to accelerate the decomposition of TiC in the Al melt at a lower temperature. So it is concluded that the transformation of TiC in Al-Si alloys are as follows.
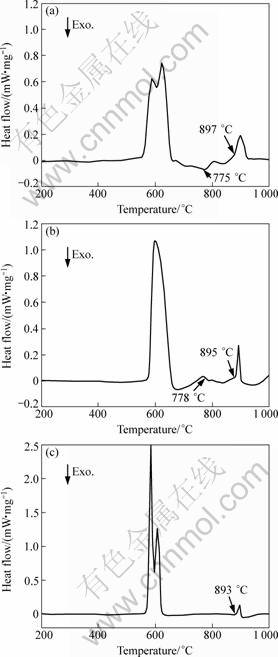
Fig. 11 DSC analyses of mixture of Al-Ti-C powder with 7% Si (a), 13% Si (b) and Al-Ti-C-7Si alloy (Sample-2) (c)
When the holding temperature is lower than 890 °C, the reaction is
![]() (4)
(4)
The work of Si is like a catalyst.
When the holding temperature is higher than 890 °C, the reaction is
![]() (5)
(5)
For reaction (5), it is deduced that the formation of Si-containing TiC in melt is the key for this process shown in Figs. 5 and 6. And it is considered that the formation of Si-containing TiC is related to the special crystal structure of TiC. It is known that the TiC particles are never found experimentally to be fully stoichiometric, but contain a lot of carbon vacancies. The concentration of vacancies can be up to one-half of the carbon lattice sites [14-15]. So the stability of TiC in the Al melt should be related to these vacancies. Thermodynamic calculations by FRAGE et al [9] indicated that the stoichiometry of the TiCx phase in Al-Ti-C system is associated with the temperature, and the TiCx formed at higher temperature would change into TiCy (x>y) at a lower temperature with the depletion of carbon. According to this study, the first procedure of the transformation in Al melt should be the out-diffusion of some carbon atoms by reaction (6). Because the vacancies can be diffusion channels, the out-diffusion of C would be, subsequently, accompanied by the incursion of other elements in the melt such as Al and Si. In this case, the influence of the incursive elements on the further out-diffusion of C is crucial for the stability of TiC and should be taken further into consideration.
![]() (6)
(6)
The possibility of the incursion of Al and Si into TiC has been analyzed using computer simulations and the results were shown in our previous work [16]. In that work, it is found that the formation energies of Al and Si substituting C vacancies in TiC are +0.98 eV and -3.89 eV, respectively. These results indicate that Si can more easily penetrate into TiC than Al. This is in accordance with the formation of Si-containing TiC in Al-Si melt. The computer simulation results also indicate that Si can further diffuse in TiC crystal lattice. Because the size of Si is much larger than that of C, it would cause serious lattice distortion which can speed the out-diffusion of surrounded C and reaction (6). Finally, the instability of TiC in Al melt is accelerated. When the holding temperature is higher than 890 °C, Si will directly react with TiC and Al to form Ti3SiC2 and Al4C3.
4 Conclusions
1) TiC particles become unstable in Al melts when Si is added and most of TiC can disappear in a short time.
2) When the melting temperature is below 890 °C, TiC will react with Al and Si to form TiAlxSiy and Al4C3 phases. But if the melt temperature is above 890 °C, TiC will react with Al and Si to form Ti3SiC2 and Al4C3.
3) It is considered that the influence of Si on the stability of TiC especially in lower temperature Al melts is due to its incursion into TiC, which will cause serious lattice distortion and then speed up the out-diffusion of surrounded C atoms from TiC.
References
[1] KENNEDY A R, KARANTZALIS A E, WYATT S M. The microstructure and mechanical properties of TiC and TiB2-reinforced cast metal matrix composites [J]. Journal of Material Science, 1999, 34: 933-940.
[2] LI P J, KANDALOVA E G, NIKITIN V I, MAKARENKO A G, LUTS A R, ZHANG Y F. Preparation of Al-TiC composites by self-propagating high-temperature synthesis [J]. Scripta Materialia, 2003, 49: 699-703.
[3] CONTRERAS A, LOPEZ V H, BEDOLLA E. Mg/TiC composites manufactured by pressureless melt infiltration [J]. Scripta Materialia, 2004, 51: 249-253.
[4] ZARRINFAR N, KENNEDY A R, SHIPWAY P H. Reaction synthesis of Cu–TiCx master-alloys for the production of copper-based composites[J]. Scripta Materialia, 2004, 50: 949-952.
[5] WANG Zhi-bin, TAN Dun-qiang, WANG Wei, YU Fang-xin. Preparation of in-situ TiC particle dispersion-strengthened copper matrix composites [J]. Foundry, 2009, 58(5): 486-488. (in Chinese)
[6] VINODKUMAR G S, MURTY B S, CHAKRABORTY M. Development of Al-Ti-C grain refiners and study of their grain refining efficiency on Al and Al-7Si alloy [J]. Journal of Alloys and Compounds, 2005, 396: 143-150.
[7] JIANG Wei-hui, HAN Xing-lin. Preparation of Al-Ti-C master alloys and their grain refining properties [J]. The Chinese Journal of Nonferrous Metals, 1998, 8(2): 268-271. (in Chinese)
[8] KENNEDY A R, WESTON D P, JONES M I. Reaction in Al–TiC metal matrix composites [J]. Materials Science and Engineering A, 2001, 316: 32-38.
[9] FRAGE N, FRUMIN N, LEVIN L, POLAK M, DARIEL M P. High temperature phase equilibria in the Al-Rich corner of the Al-Ti-C system [J]. Metallurgical and Materials Transactions A, 1998, 29: 1341-1345.
[10] SVENDSEN L, JARFORS A. Al-Ti-C phase diagram [J]. Materials Science and Technology, 1993, 9: 948-956.
[11] MOHANTY P S, GRUZIESKI J E. Grain refinement mechanisms of hypoeutectic Al-Si alloys [J]. Acta materialia, 1996, 9: 3749-3760.
[12] LOPEZ V H, SCOLES A, KENNEDY A R. The thermal stability of TiC particles in an Al-7wt%Si alloy [J]. Material Science and Engineering A, 2003, 356: 316-325.
[13] WANG Zhen-qing, LIU Xiang-fa, ZHANG Jun-yan, BIAN Xiu-fang. Reaction mechanism in the ball-milled Al-Ti-C powders [J]. Journal of Matereials Science Letters, 2003, 22: 1427-1429
[14] HUGOSSON H W, KORZHAVYI P, JANSSON U, JOHANSSON B, ERIKSSON O. Phase stability diagrams of transition metal carbides [J]. Physical Review B, 2001, 63: 165116.
[15] WILLIAMS W S. Physics of transition metal carbides [J]. Material Science and Engineering A, 1988, 105-106: 1-10.
[16] DING Hai-min, LI Hui, LIU Xiang-fa. Different elements-induced destabilization of TiC and its application on the grain refinement of Mg-Al alloys [J]. Journal of Alloys and Compounds, 2009, 48: 5285-289.
Si对TiC在铝熔体中稳定性的影响
丁海民1, 2,刘相法2
1. 华北电力大学 机械工程系,保定 071003;
2. 山东大学 材料液固结构转变与加工教育部重点实验室,济南 250061
摘 要:研究Si对TiC在铝熔体中稳定性的影响。结果表明:当一定量的Si加入到铝熔体中后,TiC变得不稳定。当熔体温度低于890 °C时,TiC会与Al及Si反应生成TiAlxSiy和Al4C3相;而当熔体温度高于890 °C时,TiC与Al和Si反应生成Al4C3及Ti3SiC2相。分析认为,Si之所以使TiC在铝熔体中变得不稳定,是由于铝熔体中的Si能够进入到TiC晶格中,并进一步扩散,形成夹杂有一定量Si的TiC。在此过程中,Si会在TiC中引起较大的晶格畸变作用,促进TiC中碳原子的迁移。
关键词:碳化钛;硅;铝-硅合金;稳定性
(Edited by YANG Hua)
Foundation item: Project (50625101) supported by the National Natural Science Fund for Distinguished Young Scholars, China; Project (51071097) supported by the National Natural Science Foundation of China; Project (11QG67) supported by the Fundamental Research Funds for the Central Universities, China
Corresponding author: DING Hai-min; Tel: +86-312-7525041; E-mail: haimin_ding@163.com
DOI: 10.1016/S1003-6326(11)60882-0


