
Effect of Mn-doping on performance of Li3V2(PO4)3/C cathode material for lithium ion batteries
ZHAI Jing1, ZHAO Min-shou1, 2, WANG Dan-dan1
1. College of Environmental and Chemical Engineering, Yanshan University, Qinhuangdao 066004, China;
2. State Key Laboratory of Metastable Material Science and Technology, Yanshan University,
Qinhuangdao 066004, China
Received 17 May 2010; accepted 8 October 2010
Abstract:
Li3V2-2/3xMnx(PO4)3(0≤x≤0.12) powders were synthesized by sol-gel method. The effect of Mn2+-doping on the structure and electrochemical performances of Li3V2(PO4)3/C was characterized by XRD, SEM, XPS, galvanostatic charge /discharge and electrochemical impedance spectroscopy(EIS). The XRD study shows that a small amount of Mn2+-doped does not alter the structure of Li3V2(PO4)3/C materials, and all Mn2+-doped samples are of pure single phase with a monoclinic structure (space group P21/n). The XPS analysis indicates that valences state of V and Mn are +3 and +2 in Li3V1.94Mn0.09(PO4)3/C, respectively, and the citric acid in raw materials was decomposed into carbon during calcination, and residual carbon exists in Li3V1.94Mn0.09(PO4)/C. The results of electrochemical measurements show that Mn2+-doping can improve the cyclic stability and rate performance of these cathode materials. The Li3V1.94Mn0.09(PO4)3/C cathode material shows the best cyclic stability and rate performance. For example, at the discharge current density of 40 mA/g, after 100 cycles, the discharge capacity of Li3V1.94Mn0.09(PO4)3/C declines from initial 158.8 mA?h/g to 120.5 mA?h/g with a capacity retention of 75.9%; however, that of the Mn-undoed sample declines from 164.2 mA?h/g to 72.6 mA?h/g with a capacity retention of 44.2%. When the discharge current is increased up to 1C, the intial discharge capacity of Li3V1.94Mn0.09(PO4)3/C still reaches 146.4 mA?h/g, and the discharge capacity maintains at 107.5 mA?h/g after 100 cycles. The EIS measurement indicates that Mn2+-doping with a appropriate amount of Mn2+ decreases the charge transfer resistance, which is favorable for the insertion/extraction of Li+.
Key words:
lithium ion batteries; cathode materials; Li3V2(PO4)3; sol-gel; doping;
1 Introduction
Since lithium iron phosphate, one kind of lithiated transition metal polyanion material based on PO43–, was first reported as a cathode material for lithium-ion batteries by PADHI et al 1997[1], the framework materials based on the phosphate polyanion have been identified as potential electroactive materials for lithium-ion batteries, and Li3V2(PO4)3 is one of them. By comparing with the commercially used LiCoO2, LiNiO2, LiMn2O4 and their derivatives, Li3V2(PO4)3 cathode material has the outstanding advantages, such as stable framework, relatively high voltage, excellent heat stability, satisfactory safety and large theoretic capacity.
However, the main drawbacks of pristine Li3V2(PO4)3 are its very low intrinsic electronic conductivity and slow transport of Li ion, which results in poor cycling stability and rate performance. This makes it difficult to utilize Li3V2(PO4)3 cathode material fully in lithium ion batteries[2-3]. To solve this problem, many efforts have been made to improve the performance of Li3V2(PO4)3 cathode material, including preparation of small radius and homogeneous particles[4-5], carbon coating[6-11] and metal cation doping[12-15]. The modification researches have improved the comprehensive electrochemical performance of Li3V2(PO4)3 to different extents. However, Mn-doping in Li3V2(PO4)3/C has not been concerned in published papers yet. In this work, the effect of Mn-doping on the structure and electrochemical performance of Li3V2(PO4)3/C was investigated.
2 Experimental
2.1 Preparation of Li3V2-2/3xMnx(PO4)3 (0≤x≤0.12)
Li3V2-2/3xMnx(PO4)3/C (x=0.03, 0.06, 0.09, 0.12) cathode materials were synthesized by sol-gel method.
V2O5·nH2O hydro-gel was firstly prepared as follows: 10% (volume fraction) H2O2 solution was slowly added to V2O5 with vigorously stirring in ice-water until a clear orange solution formed, and then brownish homogeneous V2O5 gels were obtained after 4 h at 35 °C. Aqueous solution of the stoichiometric NH4H2PO4, CH3COOLi·2H2O, (CH3COO)2Mn·4H2O and citric acid were added to the V2O5·nH2O hydro-gels, respectively. The mixture was heated with continuous stirring at 80 °C until the blue precursor was obtained. The obtained gel was dried in a vacuum oven at 80 °C, and then the powder sample was ground, pelletized and heated at 300 °C in a furnace with flowing nitrogen gas for 4 h; then temperature rose to 800 °C at a rate of 15 °C/min and kept for 5.5 h with a stream of nitrogen gas. The sample was taken out when the temperature was cooled down to room temperature, and then ground. The undoped Li3V2(PO4)3/C sample was also prepared through the same method for comparison except without addition of (CH3COO)2Mn·4H2O.
2.2 Sample characterization
X-ray diffraction pattern of the sample was carried out with 2θ between 10° and 60° by using a D/Max III diffractometer with Cu Kα radiation, at the scan speed of 2 (°)/min and voltage of 40 kV. The X-ray photoelectron spectroscopy (XPS) was obtained for the Li3V1.94Mn0.09(PO4)3/C sample by using Kratos Axis Ultra DLD spectrometer with monochromatic Al Kα radiation. The morphology of the sample was observed using the Hitachi S-4800 scanning electron microscope instrument (SEM).
2.3 Electrochemical tests
Electrochemical performances of the samples were evaluated in columned Li test cell. The cathode material was prepared by mixing the products with acetylene black and polyvinylidine fluoride (PVDF) binder at a mass ratio of 80:15:5 in N-methyl-2-pyrrolidone (NMP). The obtained slurry was coated on Al foil, dried under the infrared light and foursquare strips with dimensions of 8 mm×8 mm were cut into with active material of about 2 mg. After the strips were dried at 120 °C for 12 h in a vacuum, two-electrode electrochemical cell was assembled in a Mikrouna glove box filled with high-pure argon where the lithium metal foil was used as anode, Celgard? 2320 as separator and 1 mol/L LiPF6 in EC:DMC (1:1, volume fraction) as electrolyte, then charge/discharge test was carried out using a Neware battery tester. The electrochemical measurements were performed in the voltage range of 3.0-4.8 V at room temperature. EIS measurements were carried out in three-electrode cell by using CHI 660A electrochemical analyzer (Chenhua, Shanghai, China) with ±5 mV ac signal and a frequency range of 105-10-2 Hz and 4.8 V.
3 Results and discussion
3.1 Sample characterization
XRD patterns of Li3V2-2/3xMnx(PO4)3/C (0≤x≤0.12) are shown in Fig.1. It is obvious that all samples are of pure single phase with a monoclinic structure (space group P21/n), and the result is consistent with the published result[16]. No other phases are detected in the XRD patterns, indicating that Mn2+ ions are completely substituted into the crystal lattice of Li3V2(PO4)3, and a low dose of Mn2+-doping does not alter the basic Li3V2(PO4)3 crystal structure. The amount of carbon in Li3V2(PO4)3/C is about 4.4% in mass fraction measured by thermogravimetric analysis.
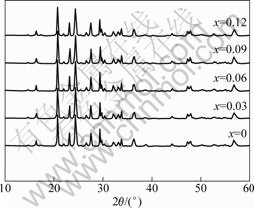
Fig.1 XRD patterns of Li3V2-2/3xMnx(PO4)3/C (0≤x≤0.12)
The XPS spectra of V2p, Mn2p and C1s in the Li3V1.94Mn0.09(PO4)3/C sample are illustrated in Fig.2. Fig.2 shows that the V2p core level fits to a single peak with a binding energy of 517.2 eV, matching well with the reported result[15], so the oxidation state of V in Li3V1.94Mn0.09(PO4)3/C is +3. The Mn2p XPS also shows a single peak with a binding energy of 640.9 eV, similar to that observed in MnO (640.6 eV)[17], so the oxidation state of Mn in Li3V1.94Mn0.09(PO4)3/C is +2. The C1s XPS shows that the peak OC—O with a binding energy of 288.4 eV is absent, while the peak C—C with a binding energy of 284.6 eV occurs in the spectra of the Li3V1.94Mn0.09(PO4)3/C. This indicates that citrate was decomposed into carbon during calcination, and residual carbon exists in Li3V1.94Mn0.09(PO4)3/C. This is beneficial to enhance the electronic conductivity for Li3V1.94Mn0.09(PO4)3/C.
The SEM images of the Li3V1.94Mn0.09(PO4)3/C and Li3V2(PO4)3/C are presented in Fig.3. The particles of them show good crystallinity. The particles of the Li3V2(PO4)3/C sample presents piece shape and are agglomerated with each other. The grains of
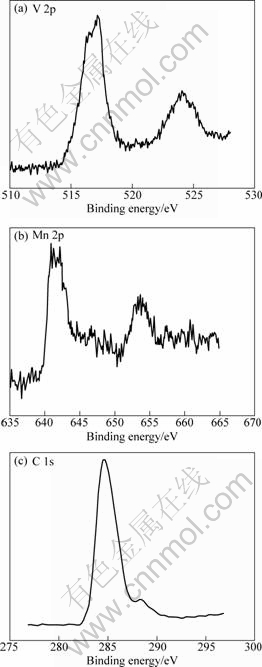
Fig.2 XPS spectra of V 2p (a), Mn 2p (b) and C 1s (c) in Li3V1.94Mn0.09(PO4)3/C
Li3V1.94Mn0.09(PO4)3/C sample are like ball shape, and the particles are smaller and more homogeneous than Li3V2(PO4)3/C sample.
3.2 Galvanostatic electrochemical measurements
The second charge/discharge curves are shown in Fig.4 for the Li/Li3V1.94Mn0.09(PO4)3 and Li/Li3V2(PO4)3 test cells at 40 mA/g current density in the range of 3.0-4.8 V at room temperature. For Li3V2(PO4)3/C sample, Fig.4(a) shows clearly four plateaus in the charge process and three plateaus in the discharge process,

Fig.3 SEM images of Li3V2(PO4)3/C (a) and Li3V1.94Mn0.09(PO4)3/C (b)
which have definite boundary. For Li3V1.94Mn0.09(PO4)3/ C sample, there are four plateaus in the charge process and two plateaus in the discharge process. Compared with the Li3V2(PO4)3/C sample, the first two discharge plateaus of Li3V1.94Mn0.09(PO4)3/C sample are merged into one. The result agrees with that of Ti-doped one[14]. Electrochemical reaction leading to multiphase reaction mechanism around plateaus usually presents new interphases, which decreases Li+ transport and accordingly affects the electrochemical performance. The more complicated the phase transition is, the more difficult the transports of Li+ is[18]. The conclusion may be drawn that there is a tendency from multiphase reaction to single-phase reaction in the charge-discharge processes by doping Mn to Li3V2(PO4)3/C. This change is beneficial to Li+ insertion/extraction and reduces the variation of cell volume during cycling, which significantly improves the cycle stability, as confirmed in Fig.5.
Fig.5 shows cycling performance of Li3V2-2/3xMnx (PO4)3(x=0, 0.03, 0.06, 0.09, 0.12) in the voltage range of 3.0-4.8 V at current density of 40 mA/g. It can be seen that the highest discharge capacity of Mn-doped samples is lower than that of undoped one; however, the cyclic stability is apparently improved except for the sample with Mn-doped 0.03 after 40 cycles, especially the cyclic stability of Li3V1.94Mn0.09(PO4)3/C is significantly
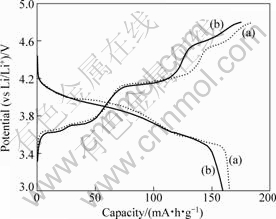
Fig.4 Second cycle charge/discharge curves of Li3V2(PO4)3/C (a) and Li3V1.94Mn0.09(PO4)3/C (b)
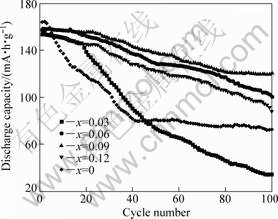
Fig.5 Cycling performance of Li3V2-2/3xMnx(PO4)3 /C (x=0, 0.03, 0.06, 0.09, 0.12)
improved. For example, after 100 cycles, the discharge capacity of Li3V1.94Mn0.09(PO4)3/C declines from initial 158.8 mA?h/g to 120.5 mA?h/g with a capacity retention of 75.9%; however, the discharge capacity of undoped one declines from initial 164.2mA?h/g to 72.6 mA?h/g with a capacity retention of 44.2%.
Rate performance of the Li3V1.94Mn0.09(PO4)3/C is shown in Fig.6. The discharge capacity decreases with increasing the cycle number at different current densities, but the change is not sharp. At different current densities of 0.2C, 0.5C and 1C, the initial discharge capacities reach 158.8, 150.8 and 146.4 mA?h/g, respectively, and the discharge capacities maintain 120.4, 110.6 and 107.5 mA?h/g after 100 cycles, respectively. From above data, we believe that the Mn-doping greatly improves the rate performance.
3.3 EIS analysis
Fig.7 shows typical Nyquist plots of the Li3V2(PO4)3/C and Li3V1.94Mn0.09(PO4)3/C samples. Prior
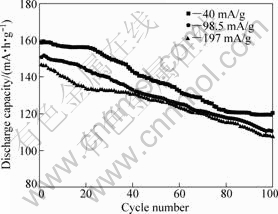
Fig.6 Discharge capacity vs cycle number for Li3V1.94Mn0.09(PO4)3/C at different current densities
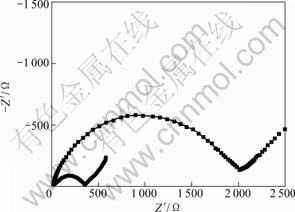
Fig.7 EIS of Li3V2(PO4)3/C and Li3V1.94Mn0.09(PO4)3/C
to the EIS measurement, the electrodes are cycled galvanostatically for 20 cycles between 3.0 and 4.8 V to ensure the stable formation of the SEI films on the surface of the electroactive particles. The EIS was then measured in the fully discharged state. As shown in Fig.7, the Nyquist plots are composed of a small intercept at high frequency, a semi-circle at high to medium frequency and a straight line at low frequency. The small intercept is almost the same (14-16 Ω) for two samples, which represents the solution resistance. The semi-circle corresponds to the charge transfer resistance and double- layer capacitance. The sloping line corresponds to the diffusion of Li+ ions in the electrode bulk, namely the Warburg impedance. It can be seen that the charge-transfer resistance on Li3V1.94Mn0.09(PO4)3/C electrode is much less than that on the undoped Li3V2(PO4)3/C. This result is similar to those previous reports[19]. The decrease of charge transfer indicates that the Mn-doping significantly can facilitate the kinetic process of electrochemical reaction on Li3V1.94Mn0.09(PO4)3/C electrode. This is favorable to overcome kinetic limit in the course of charge/discharge, enhance the depth of extraction/insertion of Li+ in active particles, reduce the difference of concentration of Li+ which lies on surface and inner of active particles, and avoid the fade of capacity because the distortion of inner crystal structure of active particles happens[20].
4 Conclusions
1) The various Mn2+-doped Li3V2-2/3xMnx(PO4)3/C (x=0, 0.03, 0.06, 0.09, 0.12) cathode materials were synthesized by sol-gel method.
2) XRD diffraction results show that a monoclinic phase is obtained, and according to XPS analysis, the valences of V and Mn in Li3V1.94Mn0.09(PO4)3/C are +3, +2, respectively. Citrate is decomposed into carbon during calcination, and residual carbon exists in Li3V1.94Mn0.09(PO4)3/C.
3) Electrochemical tests indicate that the Mn-doping into Li3V2(PO4)3/C not only presents a tendency from multiphase reaction to single-phase reaction in the charge-discharge processes, but also reduces the charge transfer resistance. As a result, the cyclic stability and rate performance of Li3V2-2/3xMnx(PO4)3/C cathode material can be effectively improved. Mn-doping is very effective for the improvement of the electrochemical performances of Li3V2(PO4)3/C.
References
[1] PADHI A K, NAJUNDASWAMY K S, GOODENOUGH J B. Phospho-olivnes as positive electrode materials for rechargeable lithium batteries [J]. J Electrochem Soc, 1997, 144(4): 1188-1194.
[2] ZHONG Sheng-kui, YIN Zhou-lan, WANG Zhi-xing, GUO Hua-jun, LI Xin-hai. Synthesis and characterization of novel cathode material Li3V2(P04)3 by carbon- thermal reduction method [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(S1): S708-S710.
[3] SAIDI M Y, BARKER J, HUANG H, SWOYER J L. ADAMSON G. Performance characteristics of lithium vanadium phosphate as a cathode material for lithium-ion batteries [J]. J Power Sources, 2003, 119-121: 266-272.
[4] LI Y Z, ZHOU Z, REN M M, GAO X P, YAN J. Improved electrochemical Li insertion performances of Li3V2(PO4)3/carbon composite materials prepared by a sol-gel route [J]. Materials Letters, 2007, 61: 4562-4564.
[5] TANG A P, WANG X Y, LIU Z M. Electrochemical behavior of Li3V2(PO4)3/C composite cathode material for lithium-ion batteries [J]. Materials Letters, 2008, 62 :1646-1648.
[6] LI Y Z, ZHOU Z, GAO X P, YAN J. A promising sol-gel route based on citric acid to synthesize Li3V2(PO4)3/carbon composite material for lithium ion batteries [J]. Electrochim Acta, 2007, 52: 4922-4926.
[7] LI Y Z, ZHOU Z, REN M M, GAO X P, YAN J. Electrochemical performance of nano-crystalline Li3V2(PO4)3/carbon composite material synthesized by a novel sol-gel method [J]. Electrochim Acta, 2006, 51: 6498-6502.
[8] CHEN Q Q, WANG J W, TANG Z, HE W C, SHAO H B, ZHANG J Q. Electrochemical performance of the carbon coated Li3V2(PO4)3 cathode material synthesized by a sol-gel method [J]. Electrochim Acta, 2007, 52: 5251-5257.
[9] CHANG C X, XIANG J F, SHI X X, HAN X X, YUAN L J, SUN J T. Rheological phase reaction synthesis and electrochemical performance of Li3V2(PO4)3/carbon cathode for lithium ion batteries [J]. Electrochim Acta, 2008, 53: 2232-2237.
[10] FU P, ZHAO Y M, DONG Y Z, AN X N, SHEN G P. Synthesis of Li3V2(PO4)3 with high performance by optimized solid-state synthesis routine [J]. J Power Source, 2006,162: 651-657.
[11] FU P, ZHAO Y M, AN X N, DONG Y Z, HOU X M. Structure and electrochemical properties of nanocarbon-coated Li3V2(PO4)3 prepared by sol-gel method [J]. Electrochim Acta, 2007, 52: 5281-5285.
[12] SATO M, OHKAWA H, YOSHIDA K, SAITO M, UEMATSU K, TODA K. Enhancement of discharge capacity of Li3V2(PO4)3 by stabilizing the orthorhombic phase at room temperature [J]. Solid-State Ionics, 2000, 135:137-142.
[13] ZHONG Sheng-kui, LIU Le-tong, JIANG Ji-qiong, LI Yan-wei, WANG Jian, LIU Jie-qun, LI Yan-hong. Preparation and electrochemical properties of Y-doped Li3V2(PO4)3 cathode materials for lithium batteries [J]. Journal of Rare Earths, 2009, 27(1): 134-137.
[14] LIU Su-qin, LI Shi-cai, HUANG Ke-long, CHEN Zhao-hui. Effect of doping Ti4+ on the structure and performances of Li3V2(PO4)3 [J]. Acta Phys Chim Sinca China, 2007, 23: 537-542.
[15] REN M M, ZHOU Z, LI Y Z, GAO X P, YAN J. Preparation and electrochemical studies of Fe-doped Li3V2(PO4)3 cathode materials for lithium-ion batteries [J]. J Power Sources, 2006, 162: 1357-1362.
[16] SAIDI M Y, BARKER J, HUANG H, SWOYER J L, ADAMSON G. Electrochemical properties of lithium vanadium phosphate as a cathode material for lithium-ion batteries [J]. Electrochem Solid State Lett A, 2002, 5: 149-151.
[17] OKU M, HIROKAWA K, IKEDA S. X-ray photoelectron spectroscopy of manganese-oxygen systems [J]. Electron Spectroscopy and Related Phenomena, 1975, 7: 465-473.
[18] PISTOIA G, ANTONINI A, ROSATI R, BELLITTO C. Effect of partial Ga3+ substitution for Mn3+ in LiMn2O4 on its behaviour as a cathode for Li cells [J]. Electroanalytical Chemistry, 1996, 410: 115-118.
[19] MI C H, ZHANG X G, ZHAO X B, LI H L. synthesis and performance of LiMn0.6Fe0.4PO4/nano-carbon webs composite cathode [J]. Materials Science and Engineering B, 2006, 129: 8-13.
[20] YANG S T, LIU Y X, YIN Y H, WANG H, WANG T. Effects of Ta ion doping on the physical and electrochemical performance of LiFePO4/C [J]. Chinese Journal of Inorganic Chemistry, 2007, 23: 1165-1168.
锰掺杂对锂离子电池正极材料Li3V2(PO4)3/C性能的影响
翟 静1,赵敏寿1, 2,王丹丹1
1. 燕山大学 环境与化学工程学院,秦皇岛 066004;
2. 燕山大学 亚稳材料制备技术与科学国家重点实验室,秦皇岛 066004
摘 要:采用溶胶-凝胶法合成Li3V2-2/3xMnx(PO4)3(0≤x≤0.12)。采用XRD、SEM、XPS、恒流充放电和电化学阻抗谱(EIS)研究Mn掺杂对Li3V2(PO4)3/C结构和电化学性能的影响。XRD研究表明:掺杂少量的Mn2+不会影响材料的结构,所有样品均具有单一相态的单斜结构(P21/n空间群)。XPS分析表明:在Li3V1.94Mn0.09(PO4)3/C中,V和Mn的化合价分别为+3和+2,原料中的柠檬酸在煅烧过程中分解成C而残留在Li3V1.94Mn0.09(PO4)3/C中。电化学测试表明:掺杂Mn改善了电极材料的循环性能和倍率性能,正极材料Li3V1.94Mn0.09(PO4)3/C表现出最好的循环稳定性和倍率性能。在40 mA/g的放电电流密度下,循环100次后,Li3V1.94Mn0.09(PO4)3/C的放电容量从158.8 mA?h/g衰减到120.5 mA?h/g,容量保持率为75.9%,而未掺杂样品的放电容量从164.2 mA?h/g衰减到72.6 mA?h/g,容量保持率为44.2%。当放电电流密度增加到1C时,Li3V1.94Mn0.09(PO4)3/C的初始放电容量仍能达到146.4 mA?h/g,循环100次后,放电容量保持为107.5 mA?h/g。EIS测试表明,掺杂适量的Mn2+减小了电荷转移阻抗,这有利于Li+的脱嵌。
关键词:锂离子电池;正极材料;Li3V2(PO4)3;溶胶-凝胶;掺杂
(Edited by YANG Hua)
Foundation item: Project (20771100) supported by the National Natural Science Foundation of China
Corresponding author: ZHAO Min-shou; Tel/Fax: +86-335-8061569; E-mail: zhaoms@ysu.edu.cn
DOI: 10.1016/S1003-6326(11)60746-2


