
Insertion/removal kinetics of lithium ion in spinel LiCuxMn2-xO4
ZENG Rong-hua(曾荣华), LI Wei-shan(李伟善), L? Dong-sheng(吕东生),
HUANG Qi-ming(黄启明), ZHAO Ling-zhi(赵灵智)
School of Chemistry and Environment, South China Normal University, Guangzhou 510006, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
The insertion/removal processes of lithium ion in spinel lithium manganese oxide(LiMn2O4) and copper doped spinel lithium manganese oxide (LiCuxMn2-xO4) on a powder microelectrode were studied by electrochemical impedance spectroscopy(EIS), cyclic voltammetry(CV) and X-ray diffractometry(XRD). The insertion/removal process of lithium ion in the spinel oxides consists of three steps: charge transfer of lithium ion on the surface of the spinel oxides, diffusion and occupation of lithium ion in the lattice of the spinel oxide. Similar to chromium, the doping of copper in spinel lithium manganese oxide results in the increase of the charge transfer resistance and the double layer capacitance for lithium insertion or removal, and the decrease of the diffusion coefficient of lithium ion in the lattice of spinel oxide. However, the insertion capacitance, a parameter reflecting the occupation of lithium ion in the lattice of the spinel oxide, is hardly influenced by the doping of copper. The influence of the doped copper on the kinetic process of lithium insertion/removal in spinel lithium manganese oxide is related to the contraction of spinel lattice.
Key words:
insertion and removal kinetics; spinel lithium manganese dioxide; doping; copper;
1 Introduction
Manganese oxides are good cathode materials for primary and secondary batteries, because they are less toxic and abundant in nature[1-2]. The most important manganese oxide is spinel lithium manganese oxide, LiMn2O4, used as the cathode materials for lithium ion battery, which is easy to prepare and has high capacity density[3-5]. However, its application is limited by its instability due to Jahn-Teller effect and dissolution of manganese into electrolyte during cycling of charging and discharging[6-8]. This problem can be solved to a great extent by substitution of other elements such as copper, cobalt and chromium for a small fraction of manganese atoms in spinel lattice[9-15].
The stability of spinel lithium manganese oxide is related to the insertion/removal kinetics of lithium ion in the spinel. Unfortunately, less knowledge is available on the effect of doped elements on the kinetics of lithium insertion and removal in the spinel oxide, which is essential for the understanding of stability improvement and for the better application of doped lithium manganese oxide. In our previous study, the effect of chromium on the insertion/removal kinetics of lithium in the spinel has been understood by using powder microelectrode[16]. The effect of copper was considered with the same method in this work.
2 Experimental
Three samples, LiMn2O4, LiCu0.05Mn1.95O4 and LiCu0.16Mn1.84O4, were synthesized by sol-gel method with CH3COOLi?2H2O, Mn(CH3COO)2, Cu(CH3COO)2? H2O, and C6H8O7?7H2O in the ratios of 1?2?0?3, 1?1.95?0.05?3 and 1?1.84?0.16?3, respectively. Aqueous solution with CH3COOLi?2H2O and Cu(CH3COO)2?H2O was added into Mn(CH3COO)2 saturated solution under stirring. Then C6H8O7?7H2O solution was added to obtain a mixture solution containing lithium, manganese and copper. The mixture solution was mediated with aqueous concentrated NH3 solution to pH=6.3 and kept at 80 ℃ under stirring until a dry precursor was obtained. The precursor was heated to 500 ℃ with 1 ℃/min and kept at 500 ℃ for 12 h to remove organic compounds and then heated to 750 ℃ with 1 ℃/min and kept at 750 ℃ for 72 h to obtain the samples. All the chemicals were in analytic grade.
XRD was conducted on a D/MAX-3A/Rigaku diffractometer with Cu Kα radiation of 30 kV, 30 mA at 12 (?)/min. The cell constant a of the spinel oxide was obtained by
a=d/(h2+k2+l2)-0.5 (1)
where h, k and l are the indexes of crystal place and d the distance between crystal planes. The volume of the spinel cell V was obtained:
V=a3 (2)
The length R/? of Mn—O and Mn—Mn was obtained with
RMn—Mn=20.5a/4 (3)
RMn—O=a(3u2-2u+0.375)0.5 (4)
where u is position constant of oxygen in the spinel lattice and equals 0.265.
Electrochemical experiments were performed with PGSTAT-30 (Autolab) in a two-electrode cell with powder microelectrode as the working electrode and metal lithium as the counter electrode and the reference electrode. The electrolyte was 1 mol/L LiPF6-EC+EMC+ DMC (mEC?mEMC?mDMC=1?1?1, Merck). The electrolyte cell was set up in a glove box under argon atmosphere. The powder microelectrode was prepared as follows [17-19]. A platinum (99.995%) wire with a diameter of 100 ?m was sealed in a glass tube to obtain a platinum microdisk electrode. The microdisk electrode was etched in a mixed concentrated acid solution for 30 min to obtain a microcavity electrode. The cavity electrode was ground on a glass plate with the mixture powder of sample and graphite (1?1, in mass fraction) to obtain the powder microelectrode.
3 Results and discussion
3.1 XRD pattern
Fig.1 shows the XRD pattern of three samples. It can be found that the diffraction patterns of three samples have characteristic of spinel crystal structure, in which lithium ions occupy the 16a positions of tetrahedra, manganese ions (Mn3+ and Mn4+) occupy the 16d positions of octahedra and oxygen ions occupy the 32e positions, the vertices of tetrahedral and octahedra. The similar diffraction patterns of spinel structure for the samples with and without doping indicate that the doped copper has entered the lattice of 16d positions to replace manganese. However, the samples with doping have MnOx and Li2MnO3 intermediate phase[9-10].

Fig.1 XRD patterns of samples: (a) LiMn2O4; (b) LiCu0.05- Mn1.95O4; (c) LiCu0.16Mn1.84O4
The cell parameters calculated with Eqns.(1)-(4) based on the diffraction peaks of Fig.1 are listed in Table 1. It can be seen from Table 1 that the cell constant, the volume and the bond length of spinel lithium manganese oxide become small due to the doping of copper and decrease as the copper contents increase, indicating that spinel cell of lithium manganese oxide is contracted due to the doping of copper. This effect can be ascribed to the stronger bond of Cu—O than that of Mn—O.
Table 1 Cell parameters of spinel samples with and without doping
3.2 Cyclic voltammogram
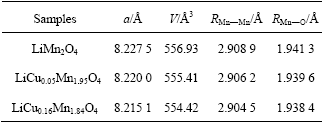
Fig.2 shows the voltammograms of three samples obtained with slow potential sweep during the first cycle. It can be seen that two steps for lithium insertion and removal in the spinel oxide can be separated significantly for the sample with and without the doping of copper. However, there are differences in the voltammograms between the samples with and without the doping of copper.
Firstly, the charging efficient and discharging efficiency are different. Charging and discharging efficiencies, the ratios of electric quantity of charging and discharging obtained by integrating forward or backward scan voltammogram, are 95%, 97%, 100% for the samples LiMn2O4, LiCu0.05Mn1.95O4 and LiCu0.16- Mn1.84O4, respectively. The charging and discharge effi-
Fig.2 Cyclic voltammograms of samples on powder microelectrode (scan rate: 80 ?V/s): (a) LiMn2O4; (b) LiCu0.05- Mn1.95O4; (c) LiCu0.16Mn1.84O4
ciencies increase with increasing copper content in the spinel oxide, indicating that irreversible capacity loss of spinel lithium manganese oxide is reduced by the doping of copper.
Secondly, the amounts of lithium insertion and removal for each of two steps, which can be obtained from the integration of the oxidation or reduction peak, are different. As shown in Fig.2(a), the amount of lithium insertion and removal at low potential is almost equal to that at high potential. However, the amount of lithium insertion and removal increases at low potential and
decreases at high potential due to the doping of copper, as shown in Figs.2(b) and (c). This suggests that the doping of copper in the spinel lithium manganese oxide changes the amount of lithium insertion and removal for each of two steps.
To understand the cyclic stability of the doped lithium manganese oxide, cyclic voltammograms of three samples were measured on powder microelectrodes with a faster scan rate. The voltammograms of the first and the 100th cycles are shown in Fig.3. It can be

Fig.3 Cyclic voltammograms of samples on powder micro- electrode (scan rate: 2mV/s): (a) LiMn2O4; (b) LiCu0.05- Mn1.95O4; (c) LiCu0.16Mn1.84O4
found from Fig.3 that the peak currents of the spinel oxide without doping decrease more quickly than those of the spinel oxides with doping. After 100 cycles, the discharge capacities, obtained by integrating the backward scanning voltammogram, become 37.7%, 13.7% and 4.4% of those at the first cycle for the samples LiMn2O4, LiCu0.05Mn1.95O4 and LiCu0.16Mn1.84-
O4, respectively. It is apparent that the cyclic stability of spinel lithium manganese oxide can be improved by the doping of copper. Similar results have been obtained for the doping of elements other than copper and this effect can be ascribed to the stability improvement of the spinel oxide by the doping of elements.
3.3 Electrochemical impedance spectroscopy
Electrochemical impedance spectroscopy was used to understand the kinetic process of lithium ion in samples. Before the EIS measurement, the powder microelectrode was charged with a constant current of 15 nA to 4.3 V and kept at 4.3 V until the current was lower than 10 pA. The measurement begun after potentiostat at each measured potential for 30 min. The frequency range used was from 100 kHz to 10 mHz with an amplitude of 5 mV.
Fig.4 shows the Nyquist plot of the sample without the doping of copper. It can be found that the Nyquist plot is composed of three part: semicircle at high frequency, straight line with a smaller slope at middle frequency and straight line with a bigger slope at low frequency. The semicircle at high frequency corresponds to a charge transfer step, which should be ascribed to the charge transfer of lithium ion on the surface of spinel oxide because there are no other electrode reactions during lithium insertion and removal in the spinel oxide. The straight line at middle frequency corresponds to a diffusion step, which can be ascribed to the diffusion of lithium in the lattice of the spinel oxide because the diffusion of lithium ion in electrolyte is fast and can be
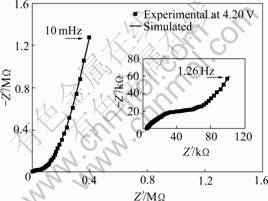
Fig.4 Nyquist plot of sample LiMn2O4 on powder micro- electrode
neglected in EIS compared with the diffusion of lithium in the lattice of the spinel. The straight line with higher slope at low frequency belongs to a capacitance behavior and it should be ascribed to the insertion/removal capacitance of lithium in the lattice of the spinel oxide. Based on these considerations, the equivalent circuit of Fig.5 can be used to describe the kinetic process of lithium in the spinel oxide.

Fig.5 Equivalent circuit describing kinetic process of lithium in spinel lithium manganese oxide on powder microelectrode
In Fig.5, Rs represents the solution resistance, Cdl the double layer capacitance, Rct the resistance of charge transfer, Zw Warburg impedance reflecting the diffusion process of lithium in the lattice of the spinel oxide and Cint insertion or desertion capacitance reflecting the occupation process of lithium in lattice of the spinel oxide. The solid line in Fig.4 is the result obtained by simulating the experimental result with the equivalent circuit of Fig.5. It can be found that the experimental result can be well fitted and thus the kinetic parameters can be easily obtained by simulation.
The electrochemical impedance of the samples with and without the doping of copper was measured under the potentials every 50 mV from 4.3 to 3.90 V. Fig.6 shows the results of three samples under 4.15, 4.10 and 4.05 V. The dots and the lines in Fig.6 represent the experimental and simulated results, respectively. It can be found that the Nyquist plots are similar for the samples with and without the doping of copper and can be well fitted by the equivalent circuit of Fig.5. The parameters of three samples under different potentials, Rs, Cdl, Rct, Zw and Cint can be obtained by simulating the measured electrochemical impedance with the equivalent circuit of Fig.5.
Fig.7 shows the dependence of charge transfer resistance Rct on the potential. It can be found that the charge transfer resistance for lithium insertion and removal in the samples with and without doping of copper is influenced by the potential and its dependence on potential is similar for the samples with and without the doping of copper. There is a minimum charge transfer resistance for all the samples, which is at the potential between the two peak potentials for two steps of lithium insertion and removal, about 4.05 V for the sample without the doping of copper and 4.1 V for the samples with the doping of copper. The charge transfer resistance

Fig.6 Nyquist plots of samples on powder microelectrode at some potential: (a) LiMn2O4; (b) LiCu0.05Mn1.95O4; (c) Li- Cu0.16Mn1.84O4
increases for all samples as the potential increases or decreases from the potential, where the charge transfer resistance is in its minimum value. By comparing the samples with and without doping of copper, it can be seen that the charge transfer resistance increases from the sample without doping to the sample with doping. The charge transfer resistance increases due to the doping of copper and as the content of doped copper in spinel oxide increases, the charge transfer resistance increases.

Fig.7 Dependence of charge transfer resistance on potential for lithium insertion and removal in samples
Fig.8 shows the dependence of double layer capacitance on the potential. It can be found that the double layer capacitance for lithium insertion and removal in the samples with and without the doping of copper is also influenced by the potential and its dependence on potential is similar for the samples with and without the doping of copper. Like the change of charge transfer resistance that has a minimum, there is a maximum double layer capacitance for all the samples, which is also at the potential between the two peak potentials for two steps of lithium insertion and removal, about 3.95 V for the sample without the doping of copper and 4.05 V for the samples with the doping of copper. The double layer capacitance decreases for all samples as the potential increases or decreases from the potential, where the double layer capacitance is in its maximum value. By comparing the samples with and without the

Fig.8 Dependence of double layer capacitance on potential for lithium insertion and removal in samples
doping of copper, it can be seen that the double layer capacitance also increases from the sample without doping to the sample with doping. There is about a decade of increase in double layer capacitance due to the doping of copper. Different from the effect of copper on the charge transfer resistance, however, the double layer capacitance of doped spinel oxide is almost independent of the content of doped copper.
To obtain the diffusion parameter of lithium in the lattice of the samples, a simplified treatment is made. The diffusion coefficient DLi of lithium in the lattice of spinel lattice can be expressed as where σ is obtained by simulating experimental electrochemical impedance with the equivalent circuit of Fig.5, A the surface area of the samples, n the electron number in the charge transfer step, T temperature, R gas constant and F Faradaic constant. Under the same temperature, A, n, T, R and F are considered to be constants and the same for all samples, thus they can be put together to be a constant κ.
![]() (5)
(5)
The dependence of diffusion coefficients of lithium in lattice of three samples obtained from Eqn.(5) is shown in Fig.9. It can be found that the diffusion coefficients of lithium in lattice of the samples with and without the doping of copper increase with increasing potential when potential is lower than 4.0 V but is less dependent on the potential when potential is higher than 4.0 V. By comparing the samples with and without the doping of copper, it can be seen that the diffusion coefficient of spinel lithium manganese oxide becomes smaller due to the doping of copper.
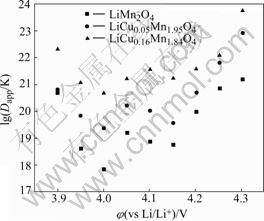
Fig.9 Dependence of diffusion coefficient on potential for lithium insertion and removal in samples
Fig.10 shows the dependence of insertion capacitance on the potential. It can be found that the insertion capacitance for the occupation of lithium in the lattice of samples with and without the doping of copper is hardly influenced by potential except for the potentials between the two peak potentials for two steps of lithium insertion and removal, where the insertion capacitance reaches a higher value. Different from the behaviors of double layer capacitance behavior, the insertion capacitance is hardly influenced by the doping of copper.

Fig.10 Dependence of insertion capacitance on potential for lithium insertion and removal in samples
The effect of doped copper on the charge transfer resistance, the double layer capacitance and the diffusion coefficient must be related to the lattice contraction of spinel lithium manganese oxide due to the doping. However, the lattice contraction of spinel lithium manganese oxide does not affect the occupation of lithium in the spinel lattice.
4 Conclusions
1) The charge transfer resistance and the double layer capacitance increase.
2) The diffusion coefficient of lithium in the lattice of spinel oxide decreases.
3) The insertion capacitance of lithium in the spinel lattice is hardly influenced by the doping of copper.
References[1] LI Wei-shan, JIANG Lin-cai, XIE Guang-yan, JIANG Xiong. Effect of H2SO4 on concentration preparation and activity of activated manganese dioxide [J]. J Power Sources, 1996, 58: 235-237.
[2] LI Wei-shan, JIANG Lin-cai, HUANG Zhong-tao. Preparation of manganese dioxide using Ag+ ions as an electrocatalyst [J]. J Power Sources, 1997, 69: 81-87.
[3] CURTIS C J, WANG J X, SCHULZ D L. Preparation and characterization of LiMn2O4 spinel nanoparticles as cathode material in secondary Li batteries [J]. J Electrochem Soc, 2004, 151: A590- A598.
[4] TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries [J]. Nature, 2001, 414: 359-367.
[5] BACH S, FARCY J, PEREIRA-RAMOS J P. An electrochemical investigation of Li intercalation in the sol-gel LiMn2O4 spinel oxide [J]. Solid State Ionics, 1998, 110: 193-198.
[6] AURBACH D, LEVI M D, LEVI E, TELLER H, MARKOVSKY B, SALITRA G, HEIDER U, HEIDER L. Common electroanalytical behavior of Li interaction processes into graphite and transition metal oxides [J]. J Electrochem Soc, 1998, 145: 3024-3034.
[7] LEVI M D, GAMULSKI K, AURBACH D, HEIDER U, OESTEN R. Evidence for slow droplet formation during cubic to tetraqonal phase transition in LiMn2O4 spinel [J]. J Electrochem Soc, 2000, 147: 25-33.
[8] AURBACH D, LEVI M D, GAMULSKI K. Capacity fading of LixMn2O4 spinel electrodes studied by XRD and electroanalytical tenchniques [J]. J Power Sources, 1999, 81/82: 472-479.
[9] EIN-ELI Y, Jr HOWARD W F. :5V cathode material [J]. Electrochemical Society Letters, 1997, 144(8): L205-L207.
[10] EIN-ELI Y, LU S H, RZEZNIK M A, MUKERJEE S, YANG X Q, MCBREEN J. LiMn2-xCuxO4 spinel (0.1≤x≤0.5): A new class of 5 cathode materials for Li batteries, II In situ measurements [J]. J Electrochem Soc, 1998, 145(10): 3383-3386.
[11] SIGALA C, SALLE A L G L , PIFARRD Y. Influence of the Cr content on the Li deinsertion behavior of the LiCryMn2-yO4 (0≤y≤1) compounds (I): Separation of bulk and superficial processes at high voltage [J]. J Electrochem Soc, 2003, 144(8): A812-A818.
[12] DAHN J R, ZHENG T, THOMAS C L. Structure and electrochemistry of Li2CrMn2-xO4 for 1.0≤x≤1.5 [J]. J Electrochem Soc, 1998, 145(3): 851-859.
[13] SONG D, IKUTA H, UCHIDA T. The spinel phases LiAlyMn2-yO4 (y=0, 1/12, 1/9, 1/6, 1/3) and Li(Al,M)1/6Mn11/6O4 (M=Cr, Co) as the cathode for rechargeable lithium batteries [J]. Solid State Ions, 1999, 117: 151-156.
[14] MOHAMEDI M, MAKINO K, DOKKO K, ITOH T, UCHIDA I. Electrochemical investigation of LiNi0.5Mn1.5O4 thin film intercalation electrodes [J]. Electrochim Acta, 2002, 48: 79-84.
[15] FEY G T K, LU C Z, KUMAR T P. Preparation electrochemical properties of and high-voltage cathode materials LiMyNi0.5-yMn1.5O4 (M=Fe, Cu, Al, Mg; y=0.0-0.4) [J]. J Power Sources, 2003, 115: 332-345.
[16] ZENG Rong-hua, LI Wei-shan, LU Dong-sheng, HUANG Qi-ming. A study on insertion/removal kinetics of lithium ion in LiCrxMn2-xO4 by using powder microelectrode [J]. J Power Sources, doi:10.1016/j.jpowsour.2007.06.120.
[17] LU Dong-sheng, LI Wei-shan. Study on electrochemical impedance spectroscopies of insertion and deinsertion of lithium ion in spinel lithium manganese oxide [J]. Acta Chimica Sinica, 2003, 61: 225-229. (in Chinese)
[18] LU Dong-sheng, LI Wei-shan. Electrochemical investigation on the interface performances of LiMn2O4/LiPF6-(EC+DEC) solution [J]. Journal of Inorganic Materials, 2004, 19: 801-808. (in Chinese)
[19] LU Dong-sheng, LI Wei-shan, ZUO Xiao-xi, YUAN Zhong-zhi, HUANG Qi-ming. A study on kinetics of Li+ insertion/de-insertion in LixMn2O4 (0≤x≤1) by electrochemical impedance spectroscopy [J]. J Phy Chem C, 2007, 111: 12067-12074
(Edited by YANG Bing)
Foundation item: Project(20373016) supported by the National Natural Science Foundation of China
Corresponding author: LI Wei-shan; Tel: +86-20-39310256; E-mail: liwsh@scnu.edu.cn


