Trans. Nonferrous Met. Soc. China 25(2015) 677-682
Synthesis of Al-SrB6 composite via powder metallurgy processing
Yucel BIROL
Department of Metallurgical and Materials Engineering, Dokuz Eylul University, Buca 35390, Izmir, Turkey
Received 1 April 2014; accepted 18 June 2014
Abstract:
The potential of powder metallurgy processing for the manufacture of Al-SrB6 composites was explored. Al4Sr particles fractured extensively during the ball milling of Al-15Sr/Al-4B powder mixtures. There was no interaction between the Al4Sr and AlB2 compounds across the section of the aluminium grains in the as-milled state. SrB6 formed, when the ball milled powder blends were subsequently annealed at sufficiently high temperatures. Ball milling for 1 h was sufficient for SrB6 to become the major constituent in powder blends annealed at 700 °C while it took 2 h of ball milling for powder blends annealed at 600 °C. Higher annealing temperatures and longer ball milling time encouraged the formation of the SrB6 compound while the latter made a great impact on the microstructural features of the Al-SrB6 composite. The SrB6 compound particles were much smaller and more uniformly distributed across the aluminium matrix grains in powder grains ball milled for 2 h before the annealing treatments at 600 °C and 700 °C.
Key words:
Al-SrB6 composites; strontium hexaboride; ball milling; powder metallurgy processing;
1 Introduction
Hexaborides of rare earth and alkaline earth metals are extremely hard, refractory solids with high chemical stabilities [1-3]. Strontium hexaboride (SrB6) is also a high melting point and stable alkali-earth metal boride that offers a low electronic work function, diverse magnetic-orders, and high neutron absorbability [4-6]. Such properties make SrB6 attractive for use in energy sources using the radioisotopes, high temperature insulation and nuclear reactor control rods. SrB6 nanoparticles dispersed in transparent acrylic sheets in aircraft windows prevent the transmittance of infrared wavelengths while still allow the transmittance of visible light to take advantage of the IR-absorbing capacity of SrB6 [7]. Single-crystal SrB6 nanowires were produced by pyrolysis of diborane (B) over SrO powders [8] to explore their potential as thermoelectric materials [9-11]. Another potential use of SrB6 is the melt treatment of aluminium foundry alloys. LI et al [12] found that SrB6 can shorten the working time of modification when studying the combined effect of Al-3Ti-B and Al-10Sr master alloy on Al-Si-9Mg alloy. SrB6 forms inevitably during the processing of aluminium foundry alloys when grain refinement and modification practices are employed [13,14].
There have been several studies in recent years that have addressed the synthesis of SrB6 in powder form. SrB6 can be formed directly from the elements by heating the well mixed powders of Sr and B [15]. The conditions appropriate for synthesizing SrB6 were optimized recently in powder formed by the reaction of SrCO3 with B4C and carbon [16]. The present work intends to produce SrB6 particles inside aluminium grains, i.e. Al-SrB6 composites, to facilitate its use in molten aluminium processing. The potential of powder metallurgy processing for the manufacture of Al-SrB6 composites was explored.
2 Experimental
Commercial Al-15Sr and Al-4B master alloy rods were pulverized to powder with a special file used specifically to prepare X-ray diffraction powder samples. 1.3 g Al-15Sr master alloy powder was mixed with 3.7 g Al-4B master alloy powder for each processing experiment. This mixing ratio is approximately equal to the stoichiometry of the SrB6 compound. Al-15Sr/ Al-4B powder mixture thus obtained was dry ball-milled for 5 to 120 min in a Spex 8000 laboratory mill using hardened steel vial and steel balls. The ball- milled powder blends were subsequently heated in a tube furnace at 600 °C and 700 °C for 1 h. The ball milled and heat treated powder blends were analyzed with X-ray diffraction (XRD), and metallographic techniques. The XRD analysis was conducted with Cu Ka radiation at a scan rate of 0.5 (°)/min in order to improve the counting frequency. Samples for metallographic analysis were mounted with a room temperature setting resin and were prepared using conventional practices. The section of the powder grains thus obtained were examined with an optical microscope.

Fig. 1 Microstructure (a) and XRD pattern (b) of Al-15Sr master alloy rods
3 Results and discussion
The microstructure of the Al-15Sr master alloy consists of coarse blocky particles dispersed across the aluminium matrix (Fig. 1(a)). These particles are identified by XRD to be Al4Sr compound particles (Fig. 1(b)). The particles of the Al-4B master alloy, on the other hand, are in the form of relatively smaller platelets (Fig. 2(a)), identified by XRD to be AlB2 compound particles (Fig. 2(b)). These master alloy rods are pulverized to powder with a special filing operation. The Al4Sr particles inside the Al-15Sr alloy have already fragmented during pulverization as evidenced by the much smaller particles inside the powder grains thus obtained (Fig. 3(a)). The AlB2 particles of the Al-4Bmaster alloy, on the other hand, are largely retained with features similar to those of the parent master alloy (Fig. 3(b)).
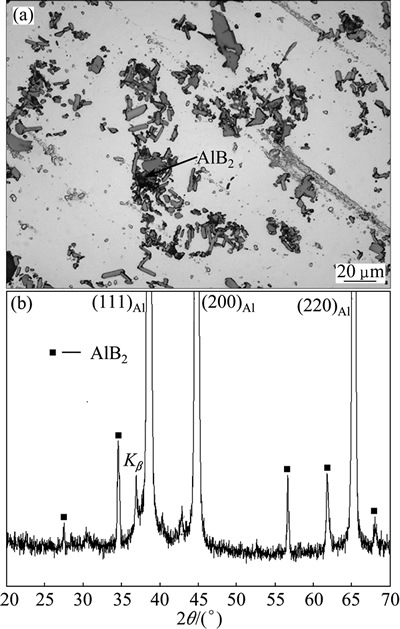
Fig. 2 Microstructure (a) and XRD pattern (b) of Al-4B master alloys
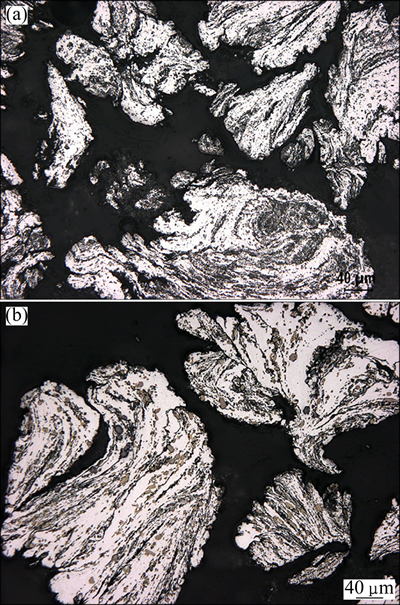
Fig. 3 Microstructures of Al-15Sr (a) and Al-4B (b) master alloy powder grains
The next step in the processing cycle is a ball milling operation. The intermixing of the Al-15Sr and Al-4B grains is very limited after 5 min of ball milling (Fig. 4(a)). Microstructural features of Al-15Sr/Al-4B powder blends sampled in the course of ball milling are typical of high-energy ball milling. Deformation, fracture and welding of powder particles trapped between colliding balls are evident. A layered structure typical of the initial stages of powder blends submitted to high energy milling has formed after ball milling for 30 min (Fig. 4(b)). The individual powder grains as well as the layers become increasingly refined with increasing ball milling time (Fig. 4(c)). This layered structure has developed further, i.e., the layers have become finer, upon further milling before it was finally replaced, after 2 h of ball milling, by a more or less homogeneous aluminium matrix with a very fine dispersion of AlB2 particles (Fig. 4(d)). Al4Sr particles, on the other hand, have fractured extensively during ball milling and could hardly be resolved in the majority of the powder grains. Some solutionizing of Sr in the aluminium matrix is also believed to have taken place. There is no evidence for any interaction between the Al4Sr and AlB2 compounds across the section of the aluminium grains in the as-milled state.
The XRD patterns of the powder blends are consistent with these microstructural features. The powder blend ball milled for 5 min reveals the reflections of the Al4Sr and AlB2 compounds in addition to those of the aluminium matrix (Fig. 5(a)). The deformation introduced by milling and the decrease in size of the Al4Sr particles by fragmentation and solutionizing in the aluminium matrix have led to the broadening of Al4Sr reflections. The samples ball milled for 30 min reveal predominantly Al reflections while reflections of the two compounds are markedly reduced owing to the incorporation, i.e., mechanical alloying, of these compouds into the aluminium grains (Figs. 5(b)-(d)). The XRD patterns of the Al-15Sr/Al-4B powder blend ball milled for 2 h finally exhibits broad Al4Sr reflections in addition to those of the aluminium matrix and rather weak AlB2 lines (Fig. 5(d)). It is inferred from the XRD patterns of the ball milled powder blends that the Al4Sr phase has been refined extensively and no SrB6 precipitation has taken place during the milling process.
A number of structural changes are identifed when the ball milled powder blends are subsequently annealed (Fig. 6). Those annealed at 600 °C evidence the gradual disappearence of the AlB2 particles and the formation of a very fine dispersion of light gray particles across the solid solution matrix grains (Figs. 6(a)-(c)). The XRD pattern of powder blends ball milled for only 5 min and then submitted to 1 h annealing treatment at 600 °C, below the melting point of the aluminium matrix, reveals weak reflections of the SrB6 compound, implying that the Sr of the Al4Sr and B of the AlB2 react to form the SrB6 compound already at 600 °C (Fig. 7(a)). There are additional weak reflections that can be assigned to Sr-B oxides, namely SrB6O10 and SrB2O4. The XRD pattern of the powder blend ball milled for 1 h before the annealing treatment at 600 °C implies a modest increase in the volume fraction of SrB6 at the expense of Sr-B oxides (Fig. 7(b)). SrB6 is clearly the predominant constituent in the powder blend ball milled for 2 h and then annealed at 600 °C (Fig. 7(c)), suggesting that the mechanical alloying introduced by ball milling facilitates the formation of SrB6 during the subsequent annealing treatment. The very fine gray particles dispersed across the aluminium matrix grains in Fig. 6(c) are evidently SrB6 particles.
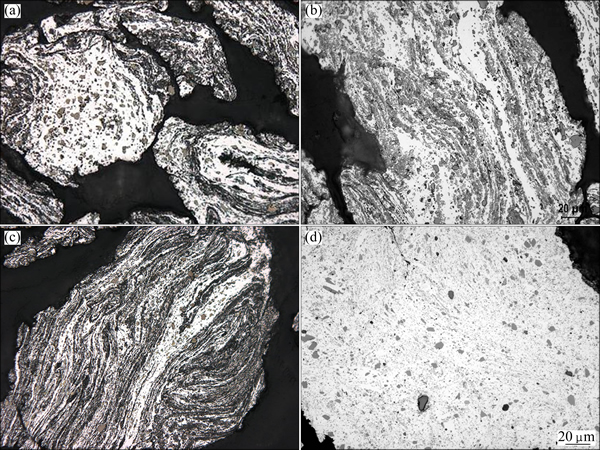
Fig. 4 Microstructures of Al-15Sr/Al-4B powder blends ball milled for 5 min (a), 30 min (b), 60 min (c) and 120 min (d)

Fig. 5 XRD patterns of Al-15Sr/Al-4B powder blends ball milled for 5 min (a), 30 min (b), 60 min (c) and 120 min (d)
Both the metallographic investigations (Figs. 6(d)- (f)) and the XRD analysis (Figs. 7(d)-(f)) of the ball milled powder blends annealed at 700 °C, i.e., above the melting point of aluminium, suggest that the reaction sequence involved is more or less the same as that identified for the annealing treatment at 600 °C.

Fig. 6 Microstructures of Al-15Sr/Al-4B powder blend samples ball milled for 5 min (a), 60 min (b) and 120 (c) min and subsequently annealed at 600 °C for 1 h, and ball milled for 5 min (d), 60 min (e) and 120 min (f) and subsequently annealed at 700 °C for 1 h
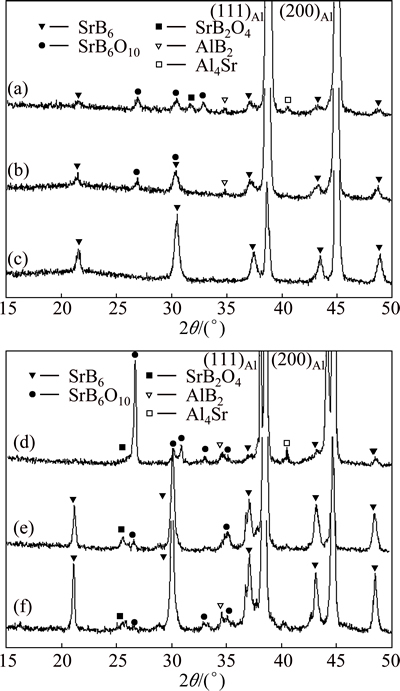
Fig. 7 XRD patterns of Al-15Sr/Al-4B powder blend samples ball milled for 5 min (a), 60 min (b) and 120 min (c) and subsequently annealed at 600 °C for 1 h, and ball milled for 5 min (d), 60 min (e) and 120 min (f) and subsequently annealed at 700 °C for 1 h
However, SrB6O10 identified to be only a minor phase in all powder blend samples annealed at 600 °C, appears to be the predominant phase in the powder blend ball milled for 5 min and subsequently annealed at 700 °C (Fig. 7(d)). The reflections of the SrB6O10 are entirely replaced with those of the SrB6 compound in powder blends ball milled for at least 1 h and then annealed at 700 °C (Figs. 7(e) and (f)). It is thus fair to conclude that SrB6O10 and SrB2O4, encountered in annealed powder blends, are intermediate transition phases. This is consistent with the work of ZHENG et al [16] who identified strontium-boron oxides, namely SrB4O7 and Sr3B2O6, to be the transition phases before SrB6 finally forms during the synthesis of SrB6 powder by the reaction of SrCO3 with B4C and carbon at temperatures as high as 1000 °C. This is a much higher temperature than that takes to synthesize SrB6 via powder metallurgy processing of Al-15Sr/Al-4B powder blends in the present work. It is evident from the metallographic analysis of powder sections that the oxide skin of the individual powder grains is a major obstacle for the fusion of the powder grains. Hence, the microstructural transformations described in the foregoing all take place inside the individual powder grains.
It is evident from Figs. 6 and 7 that Al-SrB6 composites can be readily synthesized by thermal treatment of the ball milled Al-Sr/Al-B powder blends. The composite samples thus obtained in the present work contain approximately 6% SrB6. Ball milling for 1 h is sufficient for SrB6 to become the major constituent in powder blends annealed at 700 °C while it takes 2 h of ball milling for powder blends annealed at 600 °C. This implies that the formation of the SrB6 compound is accelerated when the annealing treatment is carried out at 700 °C and higher annealing temperatures and longer ball milling times encourage the formation of the SrB6 compound. The marked difference between the microstructural features in Figs. 6(e) and (f) in spite of the similarity of the XRD patterns in Figs. 7(e) and (f) suggests that ball milling makes a great impact on the microstructural features of the Al-SrB6 composite. The SrB6 compound particles are much smaller and more uniformly distributed across the aluminium matrix grains in powder grains ball milled for 2 h before the annealing treatments at 600 °C and 700 °C. The nucleation sites of the SrB6 phase are multiplied with increasing milling time leading to the refinement of the microstructure.
4 Conclusions
1) Dry ball milling of Al-15Sr and Al-4B master alloy powders in a high energy ball milling unit and subsequent annealing of the ball milled powder blends thus obtained have produced Al-SrB6 composite.
2) SrB6 was the major constituent in powder blends annealed at 700 °C after ball milling for 1 h while it took 2 h of ball milling for powder blends annealed at 600 °C.
3) Higher annealing temperatures and longer ball milling times encouraged the formation of the SrB6 compound while the latter made a great impact on the microstructural features of the Al-SrB6 composite.
4) The SrB6 compound particles were much smaller and more uniformly distributed across the aluminium matrix grains in powder grains ball milled for 2 h before the annealing treatments at 600 °C and 700 °C.
Acknowledgements
It is a pleasure to thank F. ALAGEYIK for his help in the experimental part of the work.
References
[1] ETOURNEAU J, MERCURIO J P, HAGEMULLER P. Compounds based on octahedral b6 units: hexaborides and tetraborides [M]// MATKOVICH V I. Boron and Refractory Borides. Berlin: Springer-Verlag, 1977: 115.
[2] POST B. Refractory binary borides [M]//ADAMS R M. Boron, Metallo-Boron Compounds, and Boranes. New York: Interscience Publishers, 1964: 301.
[3] HOARD J L, HUGHES R E. Elementary boron and compounds of high boron content: Structure, properties and polymorphism [M]//MUETTERTIES E L. The Chemistry of Boron and Its Compounds. New York: Wiley, 1967: 26.
[4] SEGAWA K, TOMITA A, IWASHITA K. Electronic and magnetic properties of heavy rare-earth hexaboride single crystals [J]. J Magn Magn Mat, 1992, 104: 1233-1234.
[5] PERKINS C L, TRENARY M, TANAKA T. X-ray photoelectron spectroscopy investigation of the initial oxygen adsorption sites on the LaB6(100) surface [J]. Surf Sci, 1999, 423: L222-L228.
[6] CHEN C M, ZHOU W C, ZHANG L T. Oriented structure and crystallography of directionally solidified LaB6-ZrB2 eutectic [J]. J Amer Ceram Soc, 1998, 81: 237-240.
[7] GUERRA C. Transparent stretched acrylic sheets for aircraft window systems having controlled solar transmittance properties: US Patent Application 20090093578 [P]. 2009-09-04.
[8] JASH P, NICHOLLS A W, RUOFF R S, TRENARY M. Synthesis and characterization of single-crystal strontium hexaboride nanowires [J]. Nano Letters, 2008, 8: 3794-3798.
[9] WERHEIT H. Boron-rich solids—A chance for high efficiency high temperature thermoelectric energy conversion [J]. Mater Sci Eng B, 1995, 29: 228-232.
[10] IMAI Y, MUKAIDA M, UEDA M, WATANABE A. Screening of the possible boron-based n-type thermoelectric conversion materials on the basis of the calculated densities of states of metal borides and doped beta-boron [J]. Intermetallics, 2001, 9: 721-734.
[11] TAKEDA M, FUKUDA T, DOMINGO F, MIURA T. Thermoelectric properties of some metal borides [J]. J Solid State Chem, 2004, 177: 471-475.
[12] LI J G, MA H T, ZHANG B Q. Combined effect and its mechanism of Al-3wt.%Ti-4wt.%B and Al-10wt.%Sr master alloy on microstructures of Al-Si-Cu alloy [J]. Mater Sci Eng, 2002, 328: 169-176.
[13] BIROL Y. Interaction of grain refinement with B and modification with Sr in aluminium foundry alloys [J]. Mater Sci Tech, 2012, 28: 70-76.
[14] BIROL Y. Grain refinement and modification of Al-Si foundry alloys with B and Sr additions [J]. Mater Sci Tech, DOI: 10.1179/1743284713Y.0000000392.
[15] ROPP R C. Encyclopedia of the alkaline earth compounds [M]. Elsevier, 2013.
[16] ZHENG S Q, ZOU Z D, MIN G H, YU H S, HAN J D, WANG W T. Synthesis of strontium hexaboride powder by the reaction of strontium carbonate with boron carbide and carbon [J]. J Mater Sci Letters, 2002, 21: 313-315.
粉末冶金法制备Al-SrB6复合材料
Yucel BIROL
Department of Metallurgical and Materials Engineering, Dokuz Eylul University, Buca 35390, Izmir, Turkey
摘 要:采用粉末冶金法制备Al-SrB6复合材料。对Al-15Sr/Al-4B混合粉末进行球磨,在球磨过程中Al4Sr颗粒充分破碎。球磨态铝合金晶粒截面表明Al4Sr与AlB2之间不发生反应。混合球磨粉末经高温退火后形成SrB6。若在700 °C进行退火,混合粉末需球磨1 h可使SrB6成为主要相,而若在600 °C进行退火,混合粉末则需球磨2 h 才能使SrB6成为主要相。提高退火温度和延长球磨时间都可以促进SrB6相的形成,但后者对Al-SrB6复合材料的微观组织影响更大。经600 °C和700 °C退火处理之前,SrB6颗粒更细小,在铝基体中分布也更均匀。
关键词:Al-SrB6复合材料;SrB6;球磨;粉末冶金法
(Edited by Yun-bin HE)
Corresponding author: Yucel BIROL; Tel: +90-232301-74-51; Fax: +90-232301-74-52; E-mail: yucel.birol@deu.edu.tr
DOI: 10.1016/S1003-6326(15)63652-4
Abstract: The potential of powder metallurgy processing for the manufacture of Al-SrB6 composites was explored. Al4Sr particles fractured extensively during the ball milling of Al-15Sr/Al-4B powder mixtures. There was no interaction between the Al4Sr and AlB2 compounds across the section of the aluminium grains in the as-milled state. SrB6 formed, when the ball milled powder blends were subsequently annealed at sufficiently high temperatures. Ball milling for 1 h was sufficient for SrB6 to become the major constituent in powder blends annealed at 700 °C while it took 2 h of ball milling for powder blends annealed at 600 °C. Higher annealing temperatures and longer ball milling time encouraged the formation of the SrB6 compound while the latter made a great impact on the microstructural features of the Al-SrB6 composite. The SrB6 compound particles were much smaller and more uniformly distributed across the aluminium matrix grains in powder grains ball milled for 2 h before the annealing treatments at 600 °C and 700 °C.


