
TiB2/Ni coatings on surface of copper alloy electrode prepared by electrospark deposition
LUO Cheng1, 2, XIONG Xiang2, DONG Shi-jie3
1. Department of Materials Engineering, Hubei University of Automotive Technology, Shiyan 442002, China;
2. State Key Laboratory for Powder Metallurgy, Central South University, Changsha 410083, China;
3. School of Mechanical Engineering, Hubei University of Technology, Wuhan 430068, China
Received 22 October 2009; accepted 28 November 2010
Abstract:
In order to improve the lifespan of spot-welding electrodes used for welding zinc coated steel sheets, titanium diboride was deposited onto their surface after precoating nickel as an intermediate layer. The microstructures and phase compositions of TiB2 and Ni coatings were characterized by SEM and XRD. The coating hardness was measured using a microhardness tester. The results indicate that a satisfactory TiB2 coating is obtained as a result of the intermediate nickel layer acting as a good binder between the TiB2 coating and the copper alloy substrate. Owing to its capacity of deforming, the precoated nickel layer is dense and crack free, while cracks and pores are observed in the TiB2 coating. The hardness of the TiB2/Ni coating decreases with the increase of voltage and capacitance because of the diffusion of copper and nickel and the oxidation of the coating materials. Because of the good thermal and electrical conductivities and high hardness properties of TiB2, the deformation of the electrode with TiB2/Ni coating is reduced and its spot-welding life is by far prolonged than that of the uncoated one.
Key words:
titanium diboride; electrospark deposition; copper; electrode; nickel; coating;
1 Introduction
There has been a growing interest in improving the lifespan of spot-welding electrodes for zinc coated steel sheets. Two major methods are applied: one of which is related to matrix reinforcement by hard particles such as Al2O3, TiC, TiB2 and Zr2O3; and the other is related to surface modification by ion injection of tungsten, surface titanizing and Co brushing. Among these methods, TiC coating prepared by electrospark deposition (ESD) is the most promising and research has proven that it is capable of prolonging the lifespan of electrodes[1]. While it seems to be unavoidable that cracks and delamination occur within the superhard TiC coating[2-4]. This can be reduced by using a patent rod[5] and an intermediate nickel layer[6]. Meanwhile, it provides an opportunity for the exploitation and development of TiB2 coatings since TiB2 is superior to TiC in terms of hardness, and electrical and thermal conductivities[7], which meet the requirements of the electrode. The present work was conducted on this basis and nickel was prepared as a transitional layer between the copper substrate and the TiB2 coating to improve the poor wettability owing to its good intermiscibility with both of them.
2 Experimental
The substrate (cathode) of the ESD was a standard B nose domed flat electrode, which was precipitation strengthened and cold worked Cu-0.7Cr-0.2Zr alloy. The top surface of the substrate was cleaned with acetone before deposition. A specially sintered TiB2-based (TiB2-20Ni-7Co) ceramic rod with 7 mm in diameter and 20 mm in length, and a commercially pure nickel rod were used as the anodes. Deposition was carried out under the condition of a capacitance of 2 000 C, a voltage output of 16 V and a time of 120 s.
Phase compositions of the coatings were determined by X-ray diffraction (XRD) analysis with monochromatic Cu Kα radiation. The microstructure and compositions were determined by a scanning electron microscope (SEM) LEO1450VP equipped with an energy-dispersive spectrometer. Metallographic analysis was carried out with a Neophot32 optical microscope. The microhardness of the coating was measured with an MH-5 unit with a load of 1 N for 15 s.
3 Results and discussion
3.1 Precoated nickel
Fig.1 shows the top surface microphotographs of the precoated nickel coating with a coarse surface (Fig.1(a)). A slice in the centre of the enlarged image of Fig.1(a) is shown in Fig.1(b), which suggests the coating having a lamellar structure[8]. The slice is probably a result of the last single-pulse deposit.
The nickel coating is dense and crack free, and the cross-sectional image of the nickel coating reveals that there is no delaminating. It is a characteristic of cracks which are always observed when hard objects such as TiC, TiB2, WC and Cr7C3 are coated by ESD. It is assumed that the hard materials are more difficult to deform than soft ones such as nickel. The pulse energy affects the as-deposited coating during electrosparking, and high frequent thermal shock attacks the coating, which results in thermal stress. This phenomenon occurs because of the different thermal conductivities of the coating and substrate. For a soft coating, the stress can be released easily by deformation; however, it is difficult for a brittle material to distort. Therefore, the residual stress in a ceramic material, such as the TiB2 coating, will be accumulated to a certain degree until it is released by forming cracks. A continuous and dense nickel coating gained is helpful for being used as a beneficial transitional layer.
Optical microscopy shows that the precoated nickel layer is an uneven multi-layer structure[9], as shown in Figs.1(c) and (d). When cathode and anode materials are melted under the conditions of high temperature generated by electrosparking and mixed in the melting pool, an intermediate layer forms between the top surface of the coating and the substrate, which is a solid solution of copper (from the substrate) and nickel (from the coating). This layer is darker than the surface coating and the substrate when the specimen has been etched with 5% FeCl3 solution (Fig.1(d)). It is because that the anticorrosion properties of a solid solution are poorer than those of the pure metals of which it consists.
Fig.2 shows the XRD pattern of the top surface of precoated nickel. The major phase of the top surface is Ni, while Cu is detected too. This is an evidence of the mixing of copper with nickel in a melting pool generated by electrosparking and the diffusion of copper to the surface afterwards. It is also a character of ESD.
3.2 TiB2 coating structure
Fig.3(a)-(d) present the microphotographs of the TiB2 coating deposited on the surface of the intermediate nickel layer. It is neither even nor dense, which is different from that of the nickel coating (Fig.1(b)). Cracks are clearly seen in the enlarged image (Fig.3(b)), and a crack is even cross-sectional, as shown in Fig.3(c). Pores and delamination appear at the interface (Fig.3(c)); however, it is much less than the coating without an intermediate nickel layer. This results from the intermediate nickel layer existing as a whole between the copper substrate and the surface coating

Fig.1 SEM images of surface of pre-coated nickel (a), enlarged area of A (b), cross section (c) and cross section etched with 5% FeCl3 solution (d)
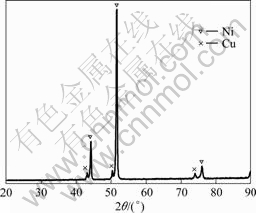
Fig.2 XRD pattern of top surface of precoated nickel
(Fig.3(d)), avoiding their direct contact.
The linear scanning results for the cross-section of TiB2/Ni coating are presented in Fig.4. Copper and nickel have diffused into the top surface of the TiB2/Ni coating. EDS shows that copper on the top surface is 8.0% and nickel is 2.3% (molar fraction). A solid atomic bonding between the coating and the matrix is formed consequently[10]. This suggests that nickel has acted as a good binder between the TiB2 coating and the copper substrate. On one hand, the atomic radii of nickel and copper are 0.124 6 nm and 0.127 8 nm, respectively, and their electronegativities are 1.91 and 1.90. Since the atomic radii and electronegativities of Ni and Cu are very
close to each other, they can resolve into each other completely. On the other hand, the wetting angle of nickel on TiB2 is 64°, indicating that nickel can wet TiB2, while TiB2 cannot wet copper due to the wetting angle of copper on TiB2 being 136°. Therefore, nickel is a good binder to connect TiB2 with copper.
Since nickel has a good wettability with TiB2, it should be rich within the coating. On the contrary, its concentration is very low, and it is not even observed in the XRD patterns of the coating, as seen in Fig.5. This happens in other researching works[3, 11]. A possible reason is that nickel may have evaporated at high temperatures generated by electrosparking and rest forms a non-crystal structure in cooling at a super high speed (10-5-10-6 K/s).
3.3 Spot-welding life
The spot-welding life of TiB2/Ni coated electrode was tested using a spot-welding machine. Lives of uncoated and TiC coated electrodes were tested as comparisons. The decreases of their lives and average height (for each weld) are illustrated in Fig.6. The hardness of the uncoated, TiC and TiB2/Ni coated electrodes are HV160, HV610 and HV520, respectively. The height decreases (wear) of the coating electrodes are reduced due to the existence of the high-hardness TiC and TiB2. Due to the low thermal conductivity, TiC and TiB2 coatings can also prevent the welding heat from transferring to the substrate of the electrodes. Therefore,
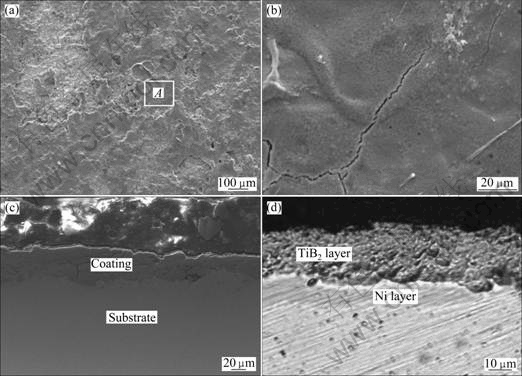
Fig.3 SEM images of TiB2/Ni coating surface (a), enlarged area of A (b), cross section (c) and optical microscopy of cross section (d)
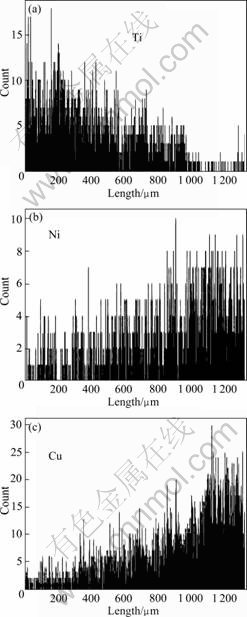
Fig.4 Linear scanning results for cross-section of TiB2/Ni coating, for Ti (a), Ni (b), Cu (d)
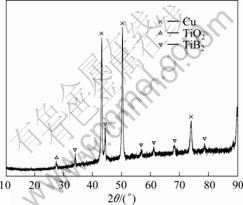
Fig.5 XRD pattern of TiB2 coating
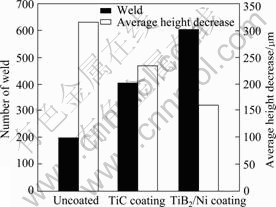
Fig.6 Spot-welding lives for electrodes with different coatings and their average height decreases
the temperature increment during the welding is lower for the TiC and TiB2/Ni coated electrodes than that for the uncoated ones. Hence, the deformation of the tip of the coated electrodes is lessened. As a result, the spot-welding lives are prelonged for the TiC and TiB2/Ni coated electrodes since the tip deformation and wear are two major factors responsible for the failure of the spot-welding electrodes[12-13]. TiB2 has better thermal and electrical conductivities than TiC (Table 1), which contributes to a lower electrical resistance (a higher welding current and stronger weld adhesion) and lower temperature rise (lower tip deformation)[14-15]. Consequently, the life of TiB2/Ni coated electrode is longer than that of TiC coated one; although the hardness of the former is a little lower than that of the latter.
Table 1 Physical properties of TiC, TiB2 and Cu

4 Conclusions
1) Nickel acts as a good binder for the coating and substrate materials. It resolves fully with the copper substrate and wets well with the TiB2 coating. The intermediate coating with phase compositions of nickel and copper is dense and crack free.
2) The TiB2/Ni coating is not dense. Cracks penetrate the whole coating and pores at the interface are observed. Copper and nickel diffuse to the coating surface and reach concentrations of 8.0% (molar fraction) and 2.3%, respectively.
3) There is no crack in the intermediate nickel coating because the residual stress can be released by deformation easily. TiB2 and TiC are difficult to deform, so the residual stress will accumulate until cracks form in the coating.
4) The spot-welding life of TiB2/Ni coated electrode is much longer than that of the uncoated and TiC coated ones.
References
[1] DONG Shi-jie, ZHOU Norman. Effect of TiC coating on electrode tip surface on electrode degradation during resistance spot welding zinc coated steel [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(2): 184-190. (in Chinese)
[2] ZHANG Xian-cheng, WU Yi-xiong, XU Bin-shi, WANG Hai-dou. Residual stresses in coating-based systems, part I: Mechanisms and analytical modeling [J]. Front Mech Eng, 2007, 2(1): 1-12.
[3] CHEN Zheng, ZHOU Yun-hong. Surface modification of resistance welding electrode by electro-spark deposited composite coatings: Part I. Coating characterization [J]. Surf Coat Technol, 2006, 201: 1503-1510.
[4] AGARWAL A, DAHOTRE N B, SUDARSHAN T S. Evolution of interface in pulsed electrode deposited titanium diboride on copper and steel [J]. Surf Eng, 1999, 15(1): 27-32.
[5] DONG Shi-jie. Rod used for electrospark depositing on the surface of spot-welding electrode: China, CN200410060704.4 [P]. 2005-03-02.
[6] ZOU Jia-sheng, ZHAO Qi-zhang, CHEN Zheng. Surface modified long-life electrode for resistance spot welding of Zn-coated steel [J]. J Mater Process Technol, 2009, 209: 4141-4146.
[7] MUNRO R G. Material properties of titanium diboride [J]. J Res Natl Inst Stand Technol, 2000, 105(5): 709-720.
[8] LESNJAK A, TUSEK J. Processes and properties of deposits in electrospark deposition [J]. Sci Technol Weld Joining, 2002, 7(6): 391-396.
[9] WANG Wei, QIAN Shi-qiang, ZHOU Xi-ying. Microstructure and properties of TiN/Ni composite coating prepared by plasma transferred arc scanning process [J]. Transaction of Nonferrous Metals Society of China, 2009, 19(5): 1180-1184.
[10] FRANGINI S, MASCI A. Intermetallic FeAl based coatings deposited by the electrospark technique: Corrosion behavior in molten (Li+K) carbonate [J]. Surf Coat Technol, 2004, 184(1): 31-39.
[11] XIE Zhi-xiong, LI Huai-jun, HUANG Hua, DONG Shi-jie. Microstructure and properties of sparking deposited composite coating on electrode surface [J]. Special Casts Nonferrous Alloys(China). 2007, 27(4): 291-293. (in Chinese)
[12] DUPUY T. The degradation of electrodes by spot welding zinc coated steels [J]. Welding in the World, 1999, 42(6): 58-68.
[13] PARKER J D, WILLIAMS N T, HOLLIDAY R J. Mechanisms of electrode degradation when spot welding coated steels [J]. Science and Technology of Welding and Joining, 1998, 3(2): 65-74.
[14] WANG Xiao-feng, SHAN Ping, HU Sheng-sun, WU Zhi-sheng, WANG Xi-bao. Effect of deep cryogenic treatment on mechanical behavior of a Cu-Cr-Zr alloy for electrodes of spot welding [J]. Rare Metals, 2005, 24(4): 392-396.
[15] HOLLIDAY R, PARKER J D, WILLIAMS N T. Electrode deformation when spot welding coated steels [J]. Welding in the World, 1995, 35(3): 160-164.
电火花沉积法在铜电极表面制备TiB2/Ni涂层
罗 成1, 2, 熊 翔2, 董仕节3
1. 湖北汽车工业学院 材料工程系, 十堰 442002;
2. 中南大学 粉末冶金国家重点实验室, 长沙 410083;
3. 湖北工业大学 机械工程学院, 武汉430068
摘 要:为了提高镀锌钢板用点焊电极的使用寿命,在铜电极表面涂覆过渡层Ni后沉积了TiB2涂层。通过SEM和XRD分析了Ni和TiB2涂层的物相和微观结构,并测试了其显微硬度。结果表明:由于材料具有良好的塑性,预涂覆的Ni涂层结构致密无裂纹,与基体Cu无分层;而TiB2涂层内存在裂纹和孔洞。通过过渡层Ni将TiB2涂层和基体Cu粘结起来,获得了较理想的涂层。随着电火花放电电容和电压的增加,TiB2的氧化程度增强,Cu和Ni大量扩散进入涂层表面,使TiB2/Ni涂层的硬度降低。由于TiB2良好的导电和导热性能及高的硬度,TiB2/Ni涂层电极在点焊时的塑性变形降低,寿命比无涂层电极和TiC涂层电极得到了明显提高。
关键词:二硼化钛; 电火花沉积; 铜; 电极; 镍; 涂层
(Edited by LI Xiang-qun)
Foundation item: Project (50575069) supported by the National Natural Science Foundation of China
Corresponding author: LUO Cheng; Tel: +86-719-8238783; E-mail: lchn69@hotmail.com
DOI: 10.1016/S1003-6326(11)60715-2


