
Effects of trace element and purification on properties of AZ80 magnesium alloy
LI Ying-ju(李应举), LUO Tian-jiao(罗天骄), YANG Yuan-sheng(杨院生)
Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China
Received 23 September 2009; accepted 30 January 2010
Abstract:
The effects of trace element Fe on the corrosion behavior of AZ80 magnesium alloy were investigated by salt spray test and electrochemical measurements. The results show that the corrosion rate decreases with decreasing the trace element Fe content in an approximately linear relation even though the amount of trace element Fe reduces to 0.000 2% (mass fraction). The electrochemical measurements show that the corrosion potential (φcorr) of the alloy with lower trace element Fe content shifts to less negative value. It is suggested that the control trace element by purification is an effective way to enhance the corrosion resistance of AZ80 magnesium alloy.
Key words:
magnesium alloy; purification; corrosion rate; trace element;
1 Introduction
Magnesium alloys have excellent properties such as lightweight, high specific strength and stiffness, machinability and recyclability[1-3] which are increasingly used in the industries. However, due to its low standard electrode potential, magnesium is one of the most reactive metals and easy to be corroded. Magnesium alloy will readily form galvanic corrosion system with another metal and even a micro-galvanic corrosion system with some secondary phases such as α-phase and/or β-phase and impurity grains[4-5] in an aqueous environment. So, the application of magnesium alloys in engineering applications is mainly limited by their unsatisfactory corrosion properties[6-8].
It is known that purification treatment[9-10] is one of the main methods to improve the corrosion resistance of magnesium alloys. High purity magnesium reveals low mass loss in salt immersion tests when the contents of Fe, Ni and Cu are reduced[11]. Deteriorative effects of these heavy impurities have been recently reconfirmed on the magnesium alloys[12].
As the most harmful element, Fe must be controlled to extremely low level, because even a very small amount of Fe will remarkably impair the corrosion resistance[9]. The deleterious effect of Fe on the corrosion resistance of magnesium alloys is attributed. Conventionally, Mn is added into the magnesium alloys to suppress the harmful effect of Fe by reducing Fe content and encapsulating Fe particles[10]. However, the usage of Mn has two typical disadvantages as Mn is likely to segregate and the ratio of Fe to Mn (a critical parameter of Mn processing) is difficult to control. B2O, Zr, ZrC1, Ti, TiO are also introduced to the magnesium alloys to reduce Fe content, while the effect of the addition of these elements to lower Fe content is not very satisfactory. The alloys still contain relatively high Fe content that is above 0.002% (mass fraction)[9]. Furthermore, the addition of these elements to the alloy will result in secondary pollution. Therefore, exploring new method to further reduce the content of impurity elements has great potential importance in the development of magnesium alloys with the required corrosion resistance.
In the present work, we try to purify magnesium alloy by vacuum melting using high purity metals. The AZ80 magnesium alloy with different Fe contents is cast. The effect of the Fe concentration on the corrosion behavior of the alloys is researched by electrochemical and gravimetric tests.
2 Experimental2.1 Material preparation
Three different-purity magnesium ingots (99.9%, 99.95%, 99.99% (mass fraction)), commercial high purity aluminum ingots (99.99%) and commercial high purity zinc ingots (99.99%) were used to prepare high purity AZ80 magnesium alloy (Mg-8.5%Al-0.5%Zn) with different Fe contents. The pre-alloyed AZ80 magnesium alloy ingots were melted in a graphite crucible (in dimensions of d60 mm×190 mm) using a vacuum furnace. The melt was refined at 700 ?C and held for about 20 min and stirred for 1 min. After being held at that temperature for another 20 min, the melt was cooled to solidify the ingot by furnace cooling. The melting and solidification processes were carried out under Ar atmosphere.
The specimens for salt spray test and electro- chemical measurement were cut in the mid portion of the alloy ingots individually. The dimension of the specimens for salt spray test was 25 mm?16 mm?10 mm, and that for the electrochemical measurement was 10 mm?10 mm?10 mm. Before the tests, the surface of the specimen was polished with SiC paper up to 2 500 grit, fine polished using 2.5 mm diamond paste, cleaned with water and alcohol, and then dried with air blower. Additionally, the electrochemical measurement specimens were covered with an epoxy resin leaving only one surface in contact with the solution.
2.2 Salt spray test
The salt spray test was performed for 48 h in the DCTC1200P dry corrosion test cabinet and the salt spray cabinet was maintained at 35 ?C. The atomization volume of fog was 1.0 to 2.0 mL of solution per hour per each 80 cm2 of horizontal collecting area. The sodium chloride concentration of the collected solution was 5% (mass fraction) and the pH of the collected solution was 6.5-7.2 according to ASTM B117.
The tested specimens were gently washed at the end of the test, and dipped in the cleaning solution of 200 g/L CrO3 and 10 g/L AgNO3 for 5-10 min to remove salt deposits from the surface, then immediately dried and finally weighed.
2.3 Electrochemical measurements
The electrochemical measurements were made using CHI660A electrochemical workstation. The corrosive medium was 3.5% (mass fraction) NaCl solution, and the three-electrode system was used with the alloy as a working electrode, the 213 model Pt electrode as auxiliary electrode and the 232 model saturated calomel electrode as reference electrode.
When the open circuit potential (OCP) became stable after the specimens were immersed into the 3.5% NaCl solution for about 15 min, the potentiodynamic polarization experiments began. The potentiodynamic polarizations scanning was performed from -300 mV relative to OCP and stopped at a potential where the corresponding current density keeps at a stationary value. The scanning rate was 5 mV/s.
2.4 Microstructures and composition analysis
The microstructures and morphologies of the alloys were characterized by a scanning electron microscope (SEM, JSM-6301F) coupled with energy dispersed spectroscope (EDS) to identify the phases composition in the alloys. The compositions of the alloys were analyzed by a inductively coupled plasma atomic emission spectrometer (ICP-AES).
3 Results and discussion
3.1 Composition of alloys
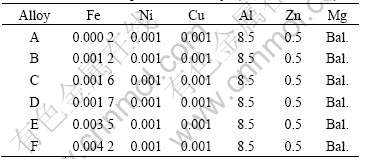
Table 1 lists the contents of the trace elements in the alloys. The results show that the contents of Ni and Cu are the same in all samples, but the Fe content changes from 0.000 2% to 0.004 2%. Because the minimum content of Fe in the most commercial magnesium alloys is 0.002%[9], these magnesium alloys listed in Table 1 are suited to study the effects of further reducing Fe content on the property of the AZ80 magnesium alloy.
Table 1 Chemical compositions of alloys (mass fraction, %)
3.2 Microstructures of alloys
The content of trace element Fe does not affect the microstructure of the cast AZ80 magnesium alloy which consists of α-Mg (the matrix), eutectic phase and β phase (Mg17Al12), as shown in Fig.1. Fig.2 shows the morphology and composition of the inclusion in the alloy. The content of the inclusion is very low and the size is about 2–3 μm. The inclusion mainly consists of magnesium and aluminum oxides. The effect of the inclusion on the corrosion property of the alloy can be neglected for its low amount and small size.
3.3 Analysis of salt spray test
The corrosion rates of the samples with different Fe contents are illustrated in Fig.3. A considerably great correlation exists between the Fe content and the corrosion rate. The corrosion rate decreases in an approximately linear relation from 0.9 to 0.2 g/(m2·h) with the Fe content in the AZ80 magnesium alloy which
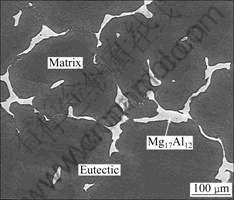
Fig.1 Microstructure of as-cast AZ80 magnesium alloy with 0.000 2% Fe
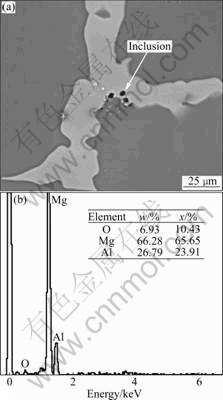
Fig.2 Morphology (a) and composition (b) of inclusion in AZ80 magnesium alloy with 0.000 2% Fe
decreases from 0.004 2% to 0.000 2%. Compared with commercial alloy AZ80 with 0.002% Fe, if Fe content decreases to 0.001 2%, the corrosion rate of the AZ80 alloy can be reduced by 40%; if Fe content decreases to 0.000 2%, the corrosion rate of the AZ80 alloy can be reduced by 140%.
The morphologies of the corrosion surfaces of the AZ80 magnesium alloys after being immersed in 5% NaCl aqueous solution for 48 h are shown in Fig.4. It can be seen that there are a few corrosion pits and the pit size is small on the surface of sample with 0.000 2% Fe, and

Fig.3 Effect of trace element Fe content on corrosion rate of AZ80 magnesium alloy in 5% NaCl at 35 ?C
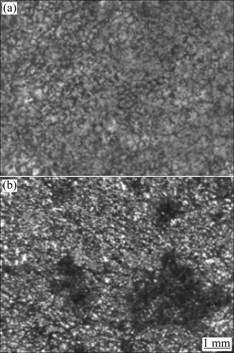
Fig.4 Corrosion morphologies of AZ80 magnesium alloy with 0.000 2% Fe (a) and 0.004 2% Fe (b) after salt spray test in 5% NaCl solution for 48 h
the corrosions in the matrix of alloy are lighter, while the corrosions at the grain boundary are serious. When the content of Fe increases to 0.004 2%, more corrosion pits are observed on the surface, the size of corrosion pit becomes larger, and the corrosions in the matrix and at grain boundary are all serious. Because the solubility of Fe in magnesium is very limited, most Fe disperses as particles in the magnesium matrix. Such dispersed Fe particles can remarkably impair the corrosion resistance of magnesium alloys[12]. Reducing the Fe content in magnesium alloys can bring about less cathode areas and can further improve the corrosion resistance of the AZ80 magnesium alloys.
3.4 Polarization curves
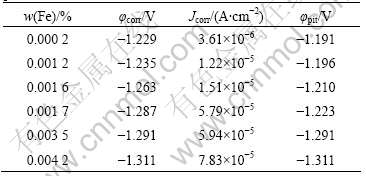
The AZ80 magnesium alloy with different Fe contents was electrochemically measured in the 3.5% NaCl solution. Fig.5 shows the potentiodynamic polarization curves and their Tafel approximation. The Tafel line shifts toward the upper left of the diagram with decreasing Fe content, which indicates an increase in the corrosion resistance and a decrease in corrosion behavior. Table 2 lists the corrosion potential (φcorr), corrosion current density (Jcorr) and pitting corrosion potential (φpit) revealed by the potentiodynamic polarization curves of alloy with different Fe contents (see Fig.5). The summarized data in Table 2 show that the corrosion potential (φcorr) of the alloy shifts to less negative value with decreasing Fe content. A good correlation is found between Jcorr values and the values of corrosion rate from salt spray test (see Fig.3). This suggests that reducing the Fe content from the normal content in the most commercial magnesium alloy can further improve the corrosion resistance of AZ80 alloys.
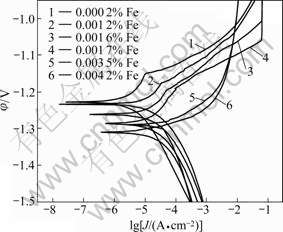
Fig.5 Potentiodynamic polarization curves of AZ80 magnesium alloys with different contents of Fe
Table 2 φcorr, Jcorr, φpit values of alloys derived from polarization curves
1) High purity AZ80 magnesium with 0.000 2% trace element Fe was cast by vacuum melting. The corrosion rate of magnesium alloy AZ80 decreases approximately linearly with decreasing trace element Fe content even though the amount of trace element Fe reduces to 0.000 2%.
2) The corrosion potential (φcorr) of alloy shifts to less negative value with the decrease of Fe content of magnesium alloy AZ80.
3) Reducing the trace element content by purification is an effective way to enhance the corrosion resistance of AZ80 magnesium alloy.
References[1] MORDIKE B L, EBERT T. Magnesium: Properties-applications- potential [J]. Materials Science and Engineering A, 2001, 302: 37-45.
[2] AGHION E, BRONFIN B, ELIEZER D. The role of the magnesium industry in protecting the environment [J]. Journal of Materials Processing Technology, 2001, 117: 381-385.
[3] FRIEDRICH H, SCHUMANN S. Research for a “new age of magnesium” in the automotive industry [J]. Journal of Materials Processing Technology, 2001, 117: 276-281.
[4] APACHITEI I, FRATILA-APACHITEI L E, DUSZCZYK J. Microgalvanic activity of an Mg-Al-Ca-based alloy studied by scanning Kelvin probe force microscopy [J]. Scripta Materialia, 2007, 57: 1012-1015.
[5] AMBAT R, AUNG N N, ZHOU W. Evaluation of microstructural effects on corrosion behaviour of AZ91D magnesium alloy [J]. Corrosion Science, 2000, 42: 1433-1455.
[6] JIA J X, SONG G L, ATRENS A. Influence of geometry on galvanic corrosion of AZ91D coupled to steel [J]. Corrosion Science, 2006, 48: 2133-2153.
[7] BENDER S, GOELLNER J, ATRENS A. Communication corrosion of AZ91 in 1N NaCl and the mechanism of magnesium corrosion [J]. Advanced Engineering Materials, 2008, 10: 583-587.
[8] HORSTEMEYER M F, YANG N, GALL K, MCDOWELL D L, FAN J, GULLETT P M. High cycle fatigue of a die cast AZ91E-T4 magnesium alloy [J]. Acta Materialia, 2004, 52: 1327-1336.
[9] GAO H T, WU G H, DING W J, LIU L F, ZENG X Q, ZHU Y P. Study on Fe reduction in AZ91 melt by B2O3 [J]. Materials Science and Engineering A, 2004, 368: 311-317.
[10] LINDER O, TERJE K A, NISANCIOGLU K. Effect of Mn additions on the corrosion behavior of mould-cast magnesium ASTM AZ91 [J]. Corrosion, 1987, 43: 291-295.
[11] HANAWALT J D, NELSON C E, PELOUBET J A. Corrosion studies of magnesium and its alloys [J]. Transactions of the Metallurgical Society of AIME, 1942, 147: 273-299.
[12] YAMAMOTO A, WATANABE A, SUGAHARA K, TSUBAKINO H, FUKUMOTO S. Improvement of corrosion resistance of magnesium alloys by vapor deposition [J]. Scripta Materialia, 2001, 44: 1039-1042.
(Edited by LONG Huai-zhong)
Foundation item: Project(2007CB613705) supported by the National Basic Research Program of China
Corresponding author: YANG Yuan-sheng; Tel: +86-24-23971728; Fax: +86-24-23844528; E-mail: ysyang@imr.ac.cn


