
Preparation and characteristics of Li2FeSiO4/C composite for cathode of
lithium ion batteries
GUO Hua-jun(郭华军), XIANG Kai-xiong(向楷雄), CAO Xuan(曹 璇),
LI Xin-hai(李新海), WANG Zhi-xing(王志兴), LI Li-ming(李黎明)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 18 June 2008; accepted 30 October 2008
Abstract:
A Li2FeSiO4/C composite cathode for lithium ion batteries was synthesized at 650 ℃ by solid-state reaction. The effects of carbon sources and carbon content on the properties of the Li2FeSiO4/C composites were investigated. The crystalline structure, morphology, carbon content and charge/discharge performance of Li2FeSiO4/C composites were determined by X-ray diffraction(XRD), scanning electron microscopy(SEM), carbon/sulfur analyzer and electrochemical measurements. As carbon content increases in the range of 5%-20%, the amount of Fe3O4 impurity phase decreases. The SEM micrographs show that the addition of the carbon is favorable for reducing the Li2FeSiO4 grain size. Using sucrose as carbon source, the Li2FeSiO4/C composite with 14.5% carbon synthesized at 650 ℃ shows good electrochemical performance with an initial discharge capacity of 144.8 mA?h/g and a capacity retention ratio of 94.27% after 13 cycles.
Key words:
lithium ion battery; cathode; Li2FeSiO4;
1 Introduction
In contrast to the high cost, toxicity, and relative instability of the cobalt-based compounds, LiFePO4 is inexpensive, nontoxic, environmentally friendly and exceptionally stable both chemically and thermally, and is regarded as likely the next cathode material for rechargeable lithium-ion batteries after the layered LiCoO2 and its derivatives[1-2]. However, this cathode suffers from the poor electronic conductivity and slow lithium ion diffusion[3-4]. In the early work, a lot of researches have been done to overcome these disadvantages, and notable enhancements in capacity and rate capability have been achieved[5-7].
Silicon is an element with natural abundance and non-toxic characteristics. Orthosilicates have intrinsic thermal stability. Recently, some reports[8-11] have indicated that silicates might be developed as a new class of cathode materials. As reported by ANTON et al[12], a further step in search for iron-based materials with aforementioned qualities seems to be the replacement of phosphorus with silicon. Nevertheless, they were found to suffer from the same drawback of poor electronic conductivity and poor rate performance as LiFePO4. The poor high rate capability has been attributed to low electronic conductivity and slow lithium ion diffusion across the two-phase interface[13-15].
In order to improve the capacity of Li2FeSiO4 cathode, Li2FeSiO4/C composite was synthesized by solid-state reaction in this work, and the crystalline structure, morphology and charge/discharge performance of the composite were investigated in detail.
2 Experimental
The precursor of Li2FeSiO4/C was prepared by a solution route. Ball milling process was applied on the precursor in the presence of carbon to coat the particles with carbon, and then Li2FeSiO4/C composite was synthesized by solid-state reaction. Lithium acetate (99%, AR), FeC2O4·2H2O (99%, AR) and Si(OC2H5)4 were used as the starting materials and were dissolved in ethanol in stoichiometric amounts. The mixture was stirred at 50 ℃ for 8 h and the ethanol was evaporated. The resulting powder was mixed with various amounts of sucrose or carbon black in ethanol then ground by ball milling. After evaporating the ethanol, the mixture was heated in a horizontal quartz tube oven at 650 ℃ for 10 h in Ar atmosphere.
The powder samples were characterized by X-ray powder diffraction analysis(XRD) using Cu Kα radiation (λ=0.154 nm) and morphology of the samples was observed by a scanning electron microscope(SEM). Elemental carbon analysis of Li2FeSiO4/C was performed by C-S 800 Determinator (Eltar, Germany).
The cathode was made from the active material, carbon black and poly (vinylidene fluoride) binder in a ratio of 80?10?10 by mass. The components were mixed as viscous slurry in N-methyl-2-pyrrolidone solvent, coated uniformly on aluminum foil and dried at 120 ℃ for at least 6 h in vacuum. The typical cathode loading was 2.5-4.0 mg/cm2. Electrochemical coin cells were assembled with lithium metal as anode, Li2FeSiO4/C as cathode, Celgard 2300 as separator and 1 mol/L LiPF6 in EC?DMC?EMC (1?1?1 by volume) as electrolyte. Cells were cycled galvanostatically between 1.5 and 4.8 V at room temperature.
3 Results and discussion
Fig.1 shows the XRD patterns of the samples prepared from the precursors with different kinds of carbon sources. The narrow diffraction peaks indicate that both samples have good crystallinity. Two impurities have been detected in the samples, and they are mainly Fe3O4 and a small amount of Li2SiO3. It is also evident that the main phases in both cases are similar. In fact, the diffraction peaks of the Li2FeSiO4 phase can be indexed by the orthorhombic unit cells of 6.271 1, 5.338 1, 4.960 7 ?, closely matching those of ANTON et al[2], which indicates that the addition of the carbon does not interfere with the main reaction, but prevents oxidation of Fe2+ and facilitates the reduction of Fe3+.
Furthermore, compared with the Li2FeSiO4/C sample prepared using carbon black as the source of carbon, the Li2FeSiO4/C sample synthesized using sucrose as the source of carbon shows much weaker diffraction peaks of Fe3O4 (2θ=34.9?), demonstrating that the content of the Fe3O4 impurity is rather lower.
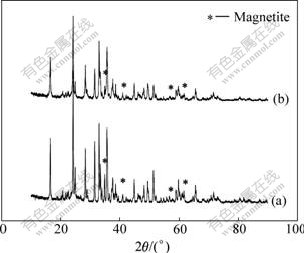
Fig.1 XRD patterns of Li2FeSiO4/C samples synthesized by using carbon black (a) and sucrose (b) as sources of carbon
Fig.2 shows the XRD patterns of Li2FeSiO4/C composites with various contents of carbon. As the carbon content increases in the range of 5%-20%, the Fe3O4 impurity phase decreases. This indicates that the addition of the sucrose can prevent the oxidation of Fe2+ and facilitate the reduction of Fe3+, and Li2FeSiO4/C composites with less impurity can be obtained when the carbon content is higher.
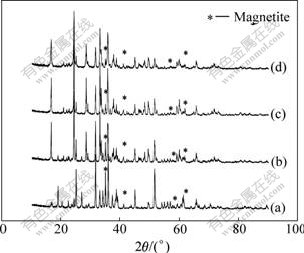
Fig.2 XRD patterns of Li2FeSiO4/C samples with various contents of carbon: (a) 5.1%; (b) 8.6%; (c) 14.5%; (d) 19.8%
Fig.3 shows the SEM images of Li2FeSiO4/C powder prepared by using different carbon sources. There are many large tight particles in the Li2FeSiO4/C sample synthesized by using carbon black as carbon source, and a lot of tiny particles are distributed among the large ones. While much smaller and more uniform particles are observed in the Li2FeSiO4/C sample obtained by using sucrose as carbon source, which is considered to be better for the diffusion of lithium in the Li2FeSiO4 particles.
Fig.4 presents the SEM images of Li2FeSiO4/C composite with different contents of carbon calcined at 650 ℃. Many Li2FeSiO4 primary particles with regular shape and disordered carbon particles can be observed in the samples. As the content of carbon increases from 5.1% to 14.5%, the size of the Li2FeSiO4 primary particles tends to reduce. It can be concluded that carbon plays an important role in hindering the growth of Li2FeSiO4 particles during calcination. The carbon particles are distributed among the reactants and Li2FeSiO4 particles, and act as a disperser and agglomeration inhibitor. Thus the excessive growth and agglomeration of Li2FeSiO4 primary particles are hampered effectively. The small particle size of the sample is favorable for Li+ diffusion because it shortens the distance of Li+ diffusion in solid particles. But when the content of carbons reaches 19.8%, obvious agglomeration is observed in the SEM micrograph, resulting in large particle size of the sample. It is due to the agglomeration of carbon particles when the carbon content is high. As a result, the reactants and Li2FeSiO4 particles are poorly dispersed and Li2FeSiO4 primary particles agglomerate dramatically.

Fig.3 SEM images of Li2FeSiO4/C powder prepared using carbon black (a) and sucrose (b) as sources of carbon
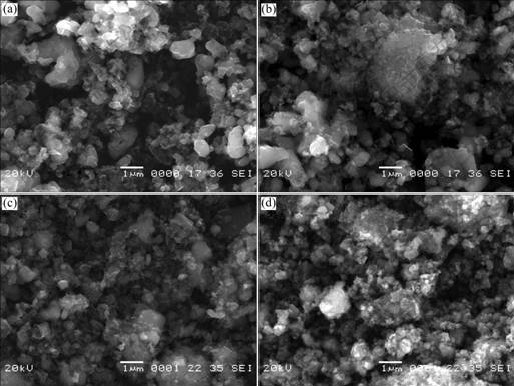
Fig.4 SEM images of Li2FeSiO4/C samples with various contents of carbon: (a) 5.1%; (b) 8.6%; (c) 14.5%; (d) 19.8%
Initial charge-discharge curves for Li2FeSiO4/C composites with various carbon contents are shown in Fig.5. The cells are cycled between 1.5 and 4.8 V using a current rate of 10 mA/g at room temperature. The specific capacity is determined without subtracting out the mass of carbon added in synthesis of Li2FeSiO4/C composite. The cell performance of the cathode materials depends on the added carbon amount in the precursor. The discharge capacity increases from 85.9 to 144.8 mA?h/g with the carbon content increasing from 5.4% to 14.5%. However, Li2FeSiO4/C composite with 19.8% carbon content delivers a discharge capacity of 103.1 mA?h/g, which is less than that of Li2FeSiO4/C composite with 14.5% carbon. It can be attributed to the serious agglomeration of the particles when the carbon content is high, as shown in Fig.4.
Fig.6 shows the cycling performance of Li2FeSiO4 cells at a current rate of 10 mA/g in the voltage range of 1.5-4.8 V. The composite with 19.8% carbon shows a capacity increase during the cycling. It is due to the serious agglomeration of the Li2FeSiO4 particles which results in the difficult activation of the cathode. The reversible capacity of other composites fades slightly, and the sample with 14.5% carbon delivers good electrochemical performance with a large initial capacity of 144.8 mA?h/g and a capacity retention ratio of 94.27% after 13 cycles.
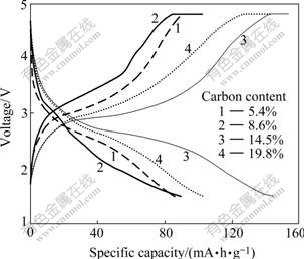
Fig.5 Initial charge-discharge curves for Li2FeSiO4/C composites at current rate of 10 mA/g
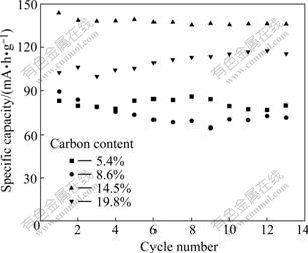
Fig.6 Cycling performance of Li2FeSiO4/C composites at current rate of 10 mA/g
4 Conclusions
1) Li2FeSiO4/C was prepared by using carbon black or sucrose as carbon source. In comparison with the Li2FeSiO4/C composite made from carbon black, the composite made from sucrose as carbon source shows lower impurities and smaller particles.
2) Using sucrose as carbon source, the effects of carbon content on the characteristics of the Li2FeSiO4/C composite were investigated and the optimized carbon content was determined to be 14.5%. The Li2FeSiO4/C composite with 14.5% carbon has good crystallinity and uniform particle distribution, and shows excellent electrochemical characteristics with a large initial reversible capacity of 144.8 mA?h/g and good cycling performance at room temperature.
References
[1] PADHI A K, NJUNDASWAMY K S, GOODENOUGH J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries [J]. J Electrochem Soc, 1997, 144: 1188-1194.
[2] PROSINI P P. LiFePO4 for lithium battery cathodes [J]. J Electrochem Soc, 2001, 145(3): A120-A121.
[3] ZHANG Bao, LI Xin-hai, WANG Xiao-qiong, WANG Zhi-xing, GUO Hua-jun. Performance enhancement of LiFePO4 for cathode material of lithium-ion battery [J]. The Chinese Journal of Nonferrous Mentals, 2006, 16(7): 1264-1168. (in Chinese)
[4] YAMADA A, CHUNG S C, HINOKUMA K. Optimized LiFePO4 for lithium battery cathodes [J]. J Electrochem Soc, 2001, 148(3): A224-A229.
[5] ZHANG Bao, LI Xin-hai, ZHU Bing-quan, WANG Zhi-xing, GUO Hua-jun. Low temperature synthesis and electrochemical properties of LiFePO4/C cathode [J]. Journal of Central South University: Science and Technology, 2006, 37(6): 505-508. (in Chinese)
[6] CHEN Z H, DAHN J R J. Reducing carbon in LiFePO4/C composite electrodes to maximize specific energy, volumetric energy and tap density [J]. J Electrochem Soc, 2002, 149(9): A1184-A1189.
[7] PARKK K S, SON J T, CHUNG H T, KIM S J, LEE C H, KANG K T, KIM H G. Surface modification by silver coating for improving electrochemical properties of LiFePO4 [J]. Solid State Communication, 2004, 129: 311-314.
[8] ZHOU F, COCOCCIONI M, KANG K, CEDER G. The Li intercalation potential of LiMPO4 and LiMSiO4 olivines with M=Fe, Mn, Co, Ni [J]. Electrochemistry Communications, 2004, 6: 1144-1148.
[9] ZAGHIB A, SALAH A A, RAVET N, MAUGER A, GENDRON F, JULIEN C M. Structural, magnetic and electrochemical properties of lithium iron orthosilicate [J]. Journal of Power Sources, 2006, 160: 1381-1386.
[10] DOMINKO R, BELE M, GABERSCEK M, MEDEN A, REMSKAR M, JAMNIK J. Structure and electrochemical performance of Li2MnSiO4 and Li2FeSiO4 as potential Li-battery cathode materials [J]. Electrochemistry Communications, 2006, 8: 217-222.
[11] LARSSON P, AHUJA R, ANTON N, THOMAS J O. An ab initio study of the Li-ion battery cathode material Li2FeSiO4 [J]. Electrochemistry Communications, 2006, 8: 797-800.
[12] ANTON N, ABOUIMRANE A, ARMAND M, GUSTAFAAON T, THOMAS J O. Electrochemical performance of Li2FeSiO4 as a new Li-battery cathode material [J]. Electrochemistry Communications, 2005, 7: 156-160.
[13] MOSKON J, DOMINKO R, KOROSEC R C, GABERSECK M, JAMNIK J. Morphology and electrical properties of conductive carbon coatings for cathode materials [J]. Journal of Power Sources, 2007, 174: 683-688
[14] ARROYO M E, ARMAND M, TARASCON J M, AMADOR U. On-demand design of polyoxianionic cathode materials based on electronegativity correlations: An exploration of the Li2MSiO4 system (M=Fe, Mn, Co, Ni) [J]. Electrochemistry Communications, 2006, 8: 1292-1298.
[15] DOMINKO R, CONTE D E, HANZEL D, GABERSCEK M, JAMNIK J. Impact of synthesis conditions on the structure and performance of Li2FeSiO4 [J]. Journal of Power Sources, 2008, 178: 842-847.
Foundation item: Project(2007CB613607) supported by the National Basic Research Program of China
Corresponding author: GUO Hua-jun; Tel: +86-731-8836633; E-mail: ghj.csu@163.com
DOI: 10.1016/S1003-6326(08)60246-0
(Edited by LI Xiang-qun)
Abstract: A Li2FeSiO4/C composite cathode for lithium ion batteries was synthesized at 650 ℃ by solid-state reaction. The effects of carbon sources and carbon content on the properties of the Li2FeSiO4/C composites were investigated. The crystalline structure, morphology, carbon content and charge/discharge performance of Li2FeSiO4/C composites were determined by X-ray diffraction(XRD), scanning electron microscopy(SEM), carbon/sulfur analyzer and electrochemical measurements. As carbon content increases in the range of 5%-20%, the amount of Fe3O4 impurity phase decreases. The SEM micrographs show that the addition of the carbon is favorable for reducing the Li2FeSiO4 grain size. Using sucrose as carbon source, the Li2FeSiO4/C composite with 14.5% carbon synthesized at 650 ℃ shows good electrochemical performance with an initial discharge capacity of 144.8 mA?h/g and a capacity retention ratio of 94.27% after 13 cycles.


