J. Cent. South Univ. Technol. (2009) 16: 0195-0200
DOI: 10.1007/s11771-009-0033-3 ![]()
Characterization of polysulfone hollow-fiber ultrafiltration membrane and its cleaning efficiency by streaming potential and flux method
QIU Yun-ren(邱运仁), MIAO Chang(缪 畅), YE Hong-qi(叶红齐)
(School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract:
Polysulfone (PS) hollow-fiber ultrafiltration membrane was characterized combined with flux and streaming potential in single electrolyte solutions. The effects of trans-membrane pressure, electrolyte concentration, ion valence and pH value of electrolyte solution on the streaming potential (SP) of the membrane were investigated. The zeta potential and surface charge density of the membrane were calculated on the basis of Helmholtz-Smoluchowski equation and Gouy-Chapmann theory. The results indicate that the valence and concentration of cation have a greater influence on the SP and surface charge density of PS membrane than those of anion, and the pH value of electrolyte solution has great effects on the SP and zeta potential of the membrane surface. Both the absolute value of the streaming potential and water flux of the adsorbed membrane decrease, compared with those of the clean membrane. The streaming potential and flux of the cleaned membrane can be completely recovered by cleaning with the mass fraction of 0.8% EDTA at pH=10.
Key words:
hollow fiber membrane; polysulfone; ultrafiltration; streaming potential; cleaning;
1 Introduction
Ultrafiltration(UF) is a pressure-driven membrane separation process widely used for separation of macromolecules or colloidal particles from liquid. Generally, UF is a separation technique mainly based on physical sieving mechanism, and the fouling and cleaning procedures can be evaluated by flux measurements. However, it cannot be taken simply as a sieve [1], for the charge on the UF membrane surface and in its pores has a great influence on the separation properties for charged solutes and particles [2]. Therefore,it is necessary to characterize the surface charge for better understanding the performance of the membrane.
Streaming potential is one of the important electrokinetic phenomena. It can be defined as the ratio of the measured electrical potential drop to the hydraulic pressure difference across a porous membrane in zero current conditions [3]. Streaming potential is easily measured and is sensitive to the change of electrolyte concentration when an electrolyte solution passes through a charged membrane by hydraulic pressure difference. Moreover, streaming potential measurements can be used to determine the membrane filtering layer charge sign and charge density [4]. ADAMCZYK et al [5] researched polyelectrolyte adsorption layers by streaming potential and particle deposition, showing that streaming potential measurements can be exploited as a sensitive tool for detecting trace amounts of adsorbed polyelectrolytes on solid surfaces. PONTI? et al [6] used the streaming potential to control fouling and cleaning procedures of UF membrane, and demonstrated that the streaming potential is a useful tool to determine membrane characteristics such as the isoelectric point (IEP), the control of the efficiency of cleaning procedures and so on.
Polysulfone (PS) membrane is widely used in the field of water treatment for its high mechanical strength, good thermal and chemical stabilities [7]. BENAVENTEA and JONSSON [8] investigated electrical and electrokinetic phenomena of a porous non- commercial PS membrane in different concentrations of NaCl and MgCl2 solutions. However, little work has been done with the aim of elucidating the surface charge properties of PS hollow fiber membrane process, especially during fouling and cleaning procedures. In this work, streaming potentials of PS hollow fiber membrane were measured in single NaCl, KCl, NaNO3, Ca(NO3)2, Mg(NO3)2 and MgSO4 solutions, and the surface charge densities were calculated according to Helmoltz- Schmolukovski equation and Gouy-Chapmann equation. The IEPs of the membranes were measured in NaNO3 and Ca(NO3)2 solutions, respectively. In addition, simultaneous streaming potential and flux measurements were used to evaluate the cleaning efficiency of the adsorbed membrane.
2 Experimental
2.1 Materials
The hollow fiber ultrafiltration membrane module was supplied by Tianjin Motian Membrane Eng & Tech Co Ltd, China. The characteristics and some available data of the membrane are summarized in Table 1. The used electrolytes were NaCl, KCl, NaNO3, Ca(NO3)2, Mg(NO3)2 and MgSO4, and all of the chemicals were analytical grade. The electrolyte solutions were prepared with distilled water, and the pH value was adjusted with 1 mol/L HCl or 1 mol/L NaOH.
Table 1 Characteristic parameters of PS hollow fiber membrane module
2.2 Measurement of streaming potential
The streaming potential was measured respectively with a pair of Ag/AgCl electrodes placed on the permeate and retentate sides close to the membrane module, and the electrodes were connected to a high impedance digital voltmeter. The electrolyte solutions were NaCl, KCl, NaNO3, Ca(NO3)2, Mg(NO3)2 and MgSO4 electrolyte solutions, all measurements were made at pH value from 2 to 9 and trans-membrane pressure from 10 to 200 kPa, and the temperature was maintained at 25 ℃. The experimental set-up for the streaming potential measurement is shown in Fig.1.

Fig.1 Experimental flow chart of ultrafiltration set-up for streaming potential measurement
2.3 Cleaning of membrane
The membrane cleaning procedure was studied respectively when the agent was single NaCl and MgSO4 solution at a concentration of 10.0 mmol/L and pH=6.8. The adsorbed membrane was obtained by filtration in electrolyte solution for 60 min, and the adsorbed membrane was cleaned by distilled water, HCl (pH=3.0) and 0.8% (mass fraction) EDTA solution (pH=10), respectively. Each cleaning agent was circulated for 20 min. After cleaning, the membranes were flushed with distilled water for 15 min. The water flux was measured and the streaming potential was measured using 1.0 mmol/L NaCl solution at pH=6.8.
3 Theory
When a gradient of electrical potential (ΔE) and pressure (Δp) are applied across a porous membrane, a volume flux (J) and an electrical current (I) will be generated according to the following equations [6]:
J=L11?p+L12?E (1)
I=L21?p+L22?E (2)
where L11, L12, L21, and L22 are coupling coefficients. Eqns.(1) and (2) are valid for any solute and solvent, and the fluxes and the gradients can be obtained from experiments. The streaming potential (φ) is defined as the ratio of the measured electrical potential drop to the hydraulic pressure difference across a porous membrane when the electric current is zero, which is obtained as follows:
![]() (3)
(3)
When pores in the membranes can be considered as charged capillaries, the zeta potential is the potential that exists in the shear plane between the bulk liquid and an envelope of water that moves with the particle. It is generally thought to lie in the diffuse layer. The thickness of this layer will be reduced when the electrolyte concentration increases. At a high electrolyte concentration the electrical double layer is compressed, which results in the Stern layer predominant in electrical double layer(EDL) [9]. The zeta potential can be calculated on the basis of the measured streaming potential data according to Helmoltz-Schmolukovski equation:
![]() (4)
(4)
where ζ is the zeta potential, V; ε0εr is the permittivity, C/(V?m); μ is the viscosity, Pa?s; and k is the conductivity of electrolyte solution, S/m. CHRISTOFOROU et al [10] thought that Eqn.(4) would be limited by k-1/rp<10
(rp is pore radius, ![]() , is Debye length,
, is Debye length,
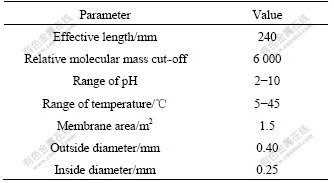
F is Faraday constant, c is the concentration of solution in mol/L, R is the molar gas constant, T is the absolute temperature). Moreover, the surface charge density (σ) can be obtained based on zeta potential by the Gouy- Chapman equation:
![]()
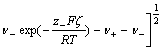 (5)
(5)
where ε is the permittivity of the solution and equals the product of ε0 and εr, v+ and v- are stoichiometric numbers of cation and anion, respectively, z+ and z- are the charge numbers of cation and anion, respectively. In the 1?1 valence solutions, the Gouy-Chapman equation can be written simply as follows:
![]() (6)
(6)
4 Results and discussion
4.1 Streaming potential
The electrical potential drop (ΔE) across the membrane was measured at different trans-membrane pressures (Δp) in the single electrolyte solution of NaCl, KCl, NaNO3, Ca(NO3)2, Mg(NO3)2 and MgSO4 at concentrations from 0.50 to 10.0 mmol/L. In Fig.2, the results show that ?E linearly decreases with the increase of Δp at the same concentration. The streaming potentials can be calculated according to the slopes of different concentrations in Fig.2, and the relationships between streaming potentials of the PS membrane and concentration in different electrolyte solutions are shown in Fig.3. The absolute values of the streaming potentials decrease with the increase of the electrolyte concentration, because the ionic strength gets higher, the dispersion layer becomes thinner, which causes more opposite counter-ions to enter the solvent layer, neutralizing part of negative charge on the surface. Moreover, the high ionic strength may make the charge of membrane surface shielded, which results in the decrease of streaming potentials.

Fig.2 Potential difference (ΔE) vs pressure difference (Δp) of PS membrane in different solutions (pH=6.8): (a) NaCl solution; (b) KCl solution; (c) NaNO3 solution; (d) Ca(NO3)2 solution; (e) Mg(NO3)2 solution; (f ) MgSO4 solution

Fig.3 Streaming potential vs concentration in different electrolyte solutions (pH=6.8)
The absolute values of streaming potentials in monovalent cation solutions are much greater than those in divalent cation solutions at the same molar concentration due to the greater ionic strength and the specific adsorption of divalent ions on the membrane surface, causing the surface charge screening [4].
4.2 Surface charge density
The surface charge density of PS membrane was calculated on the basis of the measured streaming potential according to Helmoltz-Schmolukovski equation (Eqn.(4)) and Gouy-Chapmann equations (Eqns.(5)-(6)) at the given concentration. The results are presented in Fig.4. Fig.4 shows that the surface charge density increases with the increase of electrolyte solution concentration. The results are similar to the previous research made by MOR?O et al [11] and DING et al [12]. Moreover, the surface charge densities of PS membrane in the electrolyte solutions seem to be separated by two areas: one is the monovalent cation area, in which the surface charge density increases fast with the increase of concentration, the other is the bivalent cation area, in which the surface charge density increases with concentration much more slowly than that of the former. This indicates that the valence of ions has a strong influence on the streaming potential of PS hollow fiber membrane, a cation has a stronger influence on streaming potential than an anion, and the bivalent cation has a greater influence than the monovalent cation. This may be the specific adsorption of cations, especially bivalent cations, onto the negative charged PS membrane surface [12].
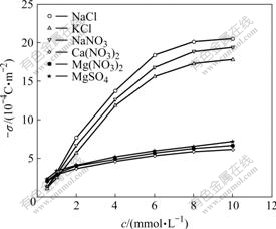
Fig.4 Surface charge density of PS membrane vs electrolyte concentration in different solutions (pH=6.8)
4.3 Isoelectric point (IEP)
The variations of streaming potentials and zeta potentials with pH value in 1.0 mmol/L NaNO3 and 1.0 mmol/L Ca(NO3)2 solutions are presented in Fig.5, respectively. The IEP of the membrane studied is 2.8-3.0 in 1.0 mmol/L NaNO3 solution, and 3.0-3.2 in 1.0 mmol/L Ca(NO3)2 solution. The IEP in 1.0 mmol/L NaNO3 solution is a little smaller than that in the same concentration of Ca(NO3)2 solution, which implies that the ionic strength takes effects on the IEP of the solution, and the bivalent cation may cause the IEP shift to a little higher position than monovalent cation does at the same concentration. This is in accordance with the precious research [11-13]. Fig.5 indicates that both the absolute value of the streaming potential and the absolute value of the zeta potential increase with the increase of pH value when pH value is greater than IEP in single NaNO3 and Ca(NO3)2 solutions. The results are in accordance with the previous ones reported by PONTI? et al [6] and MOR?O et al [11]. The absolute value of zeta potential in 1.0 mmol/L Ca(NO3)2 solution is smaller than that in 1.0 mmol/L NaNO3 solution at the same pH value, probably due to the fact that Ca2+ has a higher affinity to the membrane surface than Na+, and makes the membrane surface become less negative.
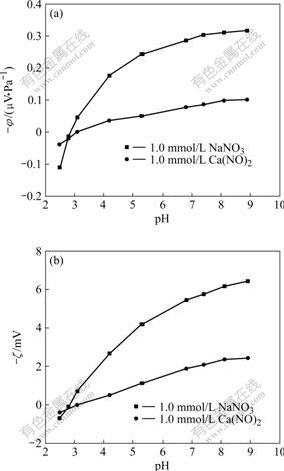
Fig.5 Streaming potential (a) and zeta potential (b) vs pH value in single NaNO3 and Ca(NO3)2 solutions
4.4 Adsorption and cleaning compared with flux and streaming potential
The water fluxes and streaming potentials of the adsorbed membrane by 10 mmol/L NaCl and 10 mmol/L MgSO4 electrolyte solutions at pH=6.8 are presented in Table 2, where RJ is the relative flux of the adsorbed membrane or the cleaned membrane, RJ = J1/J0×100%, J1 is the water flux of the adsorbed membrane or the cleaned membrane, and J0 is the water flux of the clean membrane; Rφ is the relative streaming potential of the adsorbed membrane or the cleaned membrane, Rφ=φ1/φ0×100%, φ1 is the streaming potential of the adsorbed membrane or the cleaned membrane, and φ0 is the streaming potential of the clean membrane.
Table 2 Comparison of RJ and Rφ of membranes

Table 2 shows that RJ and Rφ of the adsorbed membrane by NaCl solution is 94.20% and 90.20% respectively, RJ and Rφ of the cleaned membrane by distilled water are 95.33% and 92.20%, respectively. However, RJ and Rφ of the cleaned membrane by 0.8% EDTA (pH=10) increase up to 99.96% and 99.80%, respectively. Table 2 also shows that RJ and Rφ of the adsorbed membrane by MgSO4 solution is 92.65% and 68.20%, respectively, RJ and Rφ of the cleaned membrane by distilled water are 94.84% and 82.90% respectively. However, RJ and Rφ of the cleaned membrane by 0.8% EDTA increase up to 99.78% and 99.60%, respectively.
Thus, it can be seen that the water flux and the streaming potential of the cleaned membrane by distilled water can be slightly increased compared with those of the adsorbed membrane. However, both the water flux and the streaming potential of the cleaned membrane by 0.8% EDTA can increase up to those of the clean membrane. This can be assumed that the PS membrane is negatively charged and the cationic ions can be adsorbed to the membrane surface when electrolyte solutions flow through the PS membrane [8]. EDTA is a kind of chelating agent, which can bond Mg2+ by the formation of Mg-EDTA complexes, so EDTA is an effective cleaner for the adsorbed membranes by metallic ions [14].
Furthermore, the relative streaming potential of the adsorbed membrane is smaller than the relative water flux, that is to say, the streaming potential declines more greatly than that of water flux, especially for MgSO4-adsorbed membrane. This shows that the streaming potential is an efficient method for controlling and evaluating the cleaning procedure, especially for the membrane adsorbed by high valence cation electrolyte solutions.
5 Conclusions
(1) The absolute value of streaming potential decreases with the increase of concentration of electrolyte solution, and the surface charge densities increase with the increase of concentration of solution.
(2) The valence of ions has a great influence on the streaming potential of PS hollow fiber membrane, and a cation has a stronger influence on streaming potential than an anion. The bivalent cation has a greater influence than monovalent cation at the same concentration.
(3) The surface charge densities of PS membrane are different in different electrolyte solutions, and the surface charge density increases fast with the increase of concentration in monovalent cation electrolyte solution, while it increases slowly with the increase of concentration in bivalent cation electrolyte solution.
(4) The ionic strength takes effects on the IEP of the solution, and the bivalent cation may cause the IEP shift to a little higher position than monovalent cation does at the same concentration.
(5) Both the absolute value of the streaming potential and water flux of the adsorbed membrane decrease, compared with those of the clean membrane. The streaming potential and flux of the cleaned membrane can be completely recovered by cleaning with the mass fraction of 0.8% EDTA at pH=10.
References
[1] STRAATSMA J, BARGEMAN G, HORST H C, WESSELINGH J A. Can nanofiltration be fully predicted by a model? [J]. J Membr Sci, 2002, 198(2): 273-284.
[2] RICQ L, PIERRE A, REGGIAN J C, SERGE Z. Effects of protein on electrokinetic properties of inorganic membranes during ultra- and microfiltration [J]. J Membr Sci, 1996, 114(1): 27-38.
[3] LEVINE S, MARRIOTT J R, NEALE G. Theory of electrokinetic flow in fine cylindrical capillaries at high zeta-potentials [J]. J Coll Interf Sci, 1975, 52(1): 136-149.
[4] RICQ L, PAGETTI J. Inorganic membrane selectivity to ions in relation with streaming potential [J]. J Membr Sci, 1999, 155(1): 9-18.
[5] ADAMCZYK Z, ZEMBALA M, MICHNA A. Polyelectrolyte adsorption layers studied by streaming potential and particle deposition [J]. Coll Interf Sci, 2006, 303(2): 353-364.
[6] PONTI? M, DURAND-BOURLIER L, LEMORDANT D, LAINE J M. Control fouling and cleaning procedures of UF membranes by a streaming potential method [J]. Sep Purif Technol, 1998, 14(1): 1-11.
[7] DOYEN W, ADRIANSENS W, MOLENBERGHS B, LEYSEN R. Comparison between polysulfone, zirconia and organo-mineral membranes for use in ultrafiltration [J]. J Membr Sci, 1996, 113(2): 247-258.
[8] BENAVENTEA J, JONSSON G. Electrokinetic characterization of a microporous non-commercial polysulfone membrane: Phenomenological coefficients and transport parameters [J]. Coll Surf A: Physicochem Eng Asp, 1998, 140(1/3): 339-346.
[9] BENEFIELD L D, JUDKINS J F, WEAND B L. Process chemistry for water and wastewater treatment [M]. Englewood Cliffs, New Jersey: Prentice-Hall Inc, 1982.
[10] CHRISTOFOROU C C, WESTERMANN-CLARK G B, ANDERSON J L. The streaming potential and inadequacies of the Helmholtz equation [J]. J Coll Interf Sci, 1985,106(1): 1-11.
[11] MOR?O A I C, ALVES A M B, AFONSO M D. Concentration of clavulanic acid broths: Influence of the membrane surface charge density on NF operation [J]. J Membr Sci,2006, 281(1/2): 417-428.
[12] DING Ning, WANG Xiao-lin, WANG Jian. Electrokinetic phenomena of a polyethylene microfiltration membrane in single salt solutions of NaCl, KCl, MgCl2, Na2SO4, and MgSO4 [J]. Desalination, 2006, 192(1/3): 18-24
[13] ZHAO Yi-jian, XING Wei-hong, XU Nan-ping, WONG Fook-sin. Effects of inorganic electrolytes on zeta potentials of ceramic microfiltration membranes [J]. Sep Purif Technol, 2005, 42(2): 117-121.
[14] MASKOOKI A, KOBAYASHI T, MORTAZAVI S A, MASKOOKI A. Effect of low frequencies and mixed wave of ultrasound and EDTA on flux recovery and cleaning of microfiltration membranes [J]. Sep Purif Technol, 2008, 59(1): 67–73.
Foundation item: Project(20776161) supported by the National Natural Science Foundation of China
Received date: 2008-08-15; Accepted date: 2008-10-26
Corresponding author: QIU Yun-ren, PhD, Associate professor; Tel: +86-731-8836309; E-mail: csu_tian @mail.csu.edu.cn
(Edited by CHEN Wei-ping)
Abstract: Polysulfone (PS) hollow-fiber ultrafiltration membrane was characterized combined with flux and streaming potential in single electrolyte solutions. The effects of trans-membrane pressure, electrolyte concentration, ion valence and pH value of electrolyte solution on the streaming potential (SP) of the membrane were investigated. The zeta potential and surface charge density of the membrane were calculated on the basis of Helmholtz-Smoluchowski equation and Gouy-Chapmann theory. The results indicate that the valence and concentration of cation have a greater influence on the SP and surface charge density of PS membrane than those of anion, and the pH value of electrolyte solution has great effects on the SP and zeta potential of the membrane surface. Both the absolute value of the streaming potential and water flux of the adsorbed membrane decrease, compared with those of the clean membrane. The streaming potential and flux of the cleaned membrane can be completely recovered by cleaning with the mass fraction of 0.8% EDTA at pH=10.


