Trans. Nonferrous Met. Soc. China 23(2013) 276-282
Mechanism of mechanical activation for spontaneous combustion of sulfide minerals
Fu-qiang YANG1,2, Chao WU2
1. College of Environment and Resources, Fuzhou University, Fuzhou 350108, China;
2. School of Resources and Safety Engineering, Central South University, Changsha 410083, China
Received 9 October 2011; accepted 20 February 2012
Abstract:
In order to uncover the intrinsic reasons for spontaneous combustion of sulfide minerals, representative samples were collected from typical metal mines to carry out the mechanical activation experiment. The structures and heat behaviors of activated samples were characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD) analysis, and simultaneous thermal analysis (STA). It is found that the sulfide minerals after mechanical activation show many changes with increased specific surface areas, aggregation phenomenon, decreased diffraction peak intensity, broadened diffraction peak, declined initial temperatures of heat release and self-ignition points. A new theory for explaining the spontaneous combustion of sulfide minerals is put forward: the chemical reaction activity of sulfide minerals is heightened by all kinds of mechanical forces during the mining, and the spontaneous combustion takes place finally under proper environment.
Key words:
metal mines; mining; sulfide minerals; spontaneous combustion; mechanical activation; reaction mechanism; chemical reaction activity;
1 Introduction
When ore deposit is exploited and exposed to the air for a period of time, sulfide minerals will begin oxidizing and release lots of heat. If the rate of heat generation exceeds that of heat removal from the boundaries, the oxidizing reaction will accelerate up to auto-ignition [1]. Spontaneous combustion of sulfide minerals is a serious disaster in the mining of metal mines. Once a fire is initiated in mine, the disaster will lead to a series of environmental and safety problems [2,3].
In order to take effective measures to prevent and control the fire, the intrinsic reasons for spontaneous combustion should be uncovered. Concerning the mechanism of spontaneous combustion of sulfide minerals, there is no coincident cognition. Four main perspectives can be concluded as follows [4,5]: the physical oxygen-adsorption theory, the chemical thermodynamics mechanism, the electrochemical mechanism, and the bio-oxidation mechanism.
By the point of physical oxygen-adsorption mechanism [5,6], oxygen transports to the surfaces of broken ore particles and within ore pores, which is a heat release course and provides a basis for further chemical interaction between ore and oxygen. The chemical thermodynamical mechanism [7] regards the oxidation of sulfide ores in stope as the same chemical reaction that happens on the earth’s surface; diverse reaction modes and heat effects are presented under different environmental conditions. The theory of electrochemical mechanism [8] considers the oxidation of sulfide minerals as a process of electrochemistry; for the disfigurement of crystal lattice in sulfide minerals, tiny battery comes into being at humid environment, then oxidation reaction takes place and heat releases. By the bio-oxidation mechanism [5,9], the ore stockpile contains a large quantity of bacterium that can oxidize the sulfide minerals, which is similar to the biological metallurgy theory.
However, these perspectives just explain the reasons for oxidation of sulfide minerals from one aspect. In fact, initial stress field changes when deposit exploited, leading to the deformation and breakage of sulfide minerals. For hard metal deposit, drilling-blasting technology is used for separating and fracturing sulfide ores. Some ores need experience the second crushing for small mass fragmentation. When sulfide ores dumped by deep chute, the big ore blocks are impacted to break up during the falling. Moreover, the sulfide minerals will be comminuted by mechanical crushing in most mines. For example, the sulfide concentrates are usually processed by ball milling technics [10]. Therefore, the mining of metal deposits can be seen as a process of mechanical activation, which is concerned with mechanically induced enhancement of the chemical reactivity [11].
In this work, the authors try to conduct mechanical activation test of typical sulfide minerals, and analyze the changes in physico-chemical properties to uncover the intrinsic reasons for spontaneous combustion.
2 Experimental
2.1 Sample preparation
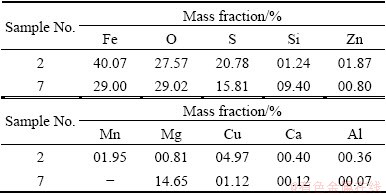
Two representative samples (2, 7) were obtained from Yingiagou Iron Sulfide Mine and Dongguashan Copper Mine in China. The oxidized surfaces of samples were removed, and particle diameter was broken into about 0.2 mm by hand. The chemical compositions of samples are given in Table 1. It indicates that the sulfur and iron contents of two samples are high and each sample contains a variety of impurities. The XRD results display that sample 2 is mainly composed of FeS2 (pyrite), FeCO3 (siderite), and SiO2 (quartz); sample 7 mostly contains pyrrhotite (Fe1-xS), FeS2 (pyrite), copper aluminium sulfide (CuAlS2), Fe3O4 (magnetite), and valleriite(Cu2Fe4S7).
Table 1 Chemical compositions of two samples
2.2 Mechanical activation and characterization
The mechanochemical study refers to high intensity grinding mills and the methods for detecting the mechanochemical effects. There are many kinds of grinding mills delivering huge amount of energy for ore breakage, such as oscillating mill, jet mill, vibration mill and planetary mill [12]. Scanning electron microscopy (SEM) analysis, X-ray diffraction (XRD), simultaneous thermal analysis (STA), X-ray photoelectron(XPS), infrared spectroscopy (IR), and laser particle analyzer usually serve as reliable tools for examining the effects of mechanical activation on sulfide minerals [13-15].
In this experiment, two samples were activated in a planetary mill. Forty grams unactivated sulfide minerals were added into a stainless steel vessel. A mixture of 2 stainless steel balls of 20 mm in diameter, 10 balls of 15 mm in diameter, and 40 balls of 10 mm in diameter was used as the grinding media, with powder-to-media mass ratio of 1:8. The whole dry grinding tests were carried out in air atmosphere where the planetary mill (KQM, made in Nanjing, China) was kept closed during the grinding process. Milling was performed for 15, 30, 45 and 60 min at a planet carrier speed of 160 r/min, respectively.
The granulometric surface areas of the activated samples were determined by corresponding particle size distribution data measured on a Mastersizer 2000 laser diffraction particle size analyzer (Malvern, Great Britain). The distilled water was used as a dispersing agent.
XRD measurements were carried out by X-ray diffraction on the diffractometer (Rigaku D/max 2500 PC, Japan) using Cu Kα radiation (λ=1.54  , voltage 40 kV, current 20 mA) with time constant 0.5 s, scanning speed 2 (°)/min.
, voltage 40 kV, current 20 mA) with time constant 0.5 s, scanning speed 2 (°)/min.
Scanning electron microscopy in combination with EDAX was performed with an Quanta 200 (FEI, Hong Kong) for comparing the photographs of activated samples.
Simultaneous thermal analysis (STA) was conducted using a NETZSCH STA 449C instrument for mastering the heat behaviors of diverse samples.
3 Results and discussion
3.1 Structural changes of samples after mechanical activation
The photomicrographs of non-activated and mechanically activated samples at different grinding periods are shown in Figs. 1 and 2. It can be seen that the sulfide minerals are broken into rough particles by grinding for different time. For non-activated sample, the surface is flat and clean, with clear figures and obvious boundary between particles. While for the activated samples, it is clear that an aggregated structure is formed during the milling process, with the size distribution of particles in a large range; small debris is sticking on the larger particles. For the plane of incomplete cleavage of samples, it is easy to break into small particles [15]. Mild aggregation phenomenon is observed at 15 min, whilst more small particles appear sticking on the larger particles and massive aggregation is presented after 60 min. This phenomenon is attributed to the tendency of activated minerals to reduce their surface free energies [16]. For different mineralogical compositions, aggregation is more pronounced for sample 7 than sample 2 at the same grinding time.

Fig. 1 SEM photographs of sample 2 after grinding for different time
Obviously, the process of mechanical activation results in an increase in the number of particles and generation of fresh surfaces. As shown in Fig. 3, the specific surface areas of two activated samples in air atmosphere increase with increasing milling time.
The XRD peak (104) of mechanically activated sample 2 and peak (001) of activated sample 7 are shown in Figs. 4 and 5, respectively. It can be seen that the intensity of diffraction peak decreases and the diffraction peak broadens continuously with increasing milling time. These observations demonstrate a rise in lattice deformation, decrease in crystallite size and reduction in symmetry of unit cell [15,17].

Fig. 2 SEM photographs of sample 7 after grinding for different time

Fig. 3 Relationship between specific surface area and grinding time
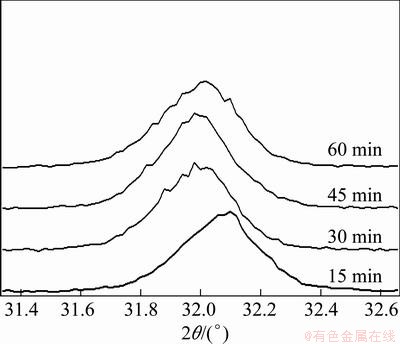
Fig. 4 Peak (104) of XRD pattern of sample 2 after grinding for different time
Although new mineralogical composition is not identified in XRD patterns of two mechanically activated samples, it doesn’t suggest that sulfide minerals do not undergo chemical reaction during the milling. According to POURGHAHRAMANI and FORSSBERG [18], the phases below 2% can not be shown by XRD method. In fact, a mechanically stimulated phase transformation has been identified by EYMERY and YLLI [19], who reported the observation of a phase transformation from pyrite (FeS2) to szomolnokite (FeSO4·H2O) by the mechanical action of ball-milling in air atmosphere. It has been proved that the formation of elemental sulfur during mechanical activation leads to the appearance of reactive sites on surface of activated sulfide minerals [15]. A part of applied energy was stored in sulfide minerals during mechanically stressing of divided solid, which was used to bend or break the crystalline particles [18,19]. The phase transformations can be attributed to the local high temperatures and pressures on contact surface of mechanically activated sulfide minerals with the presence of volume defects [20,21].

Fig. 5 Peak (001) of XRD spectra of sample 7 after grinding for different time
3.2 Changes on TG-DTG-DSC curves of samples after mechanical activation
In order to understand the thermal behaviors of samples after mechanical activation, simultaneous thermal analysis experiments were conducted by a simultaneous thermal analysis instrument. Synthetic air (20.5% O2 in N2) was used as the pure gas with a flow rate of 20 mL/min. Each sample with a mass of 9 mg was tested at the heating rate of 15 °C/min from 25 °C to 800 °C.
The corresponding curves of TG-DTG-DSC analysis for the chemical reaction of sample 2 are shown in Figs. 6-8, which indicate the complexity of the whole reaction process. The obvious mass loss and exothermic peaks can be found. It is clear that the TG curves of mechanically activated samples move down with increasing milling time, and the initial reaction temperature decreases. A new pronounced mass loss peak for the mechanically activated samples between 600 and 750 °C can be found from the DTG curves. The temperature to the maximum reaction rate can be considered the auto-ignition point of sulfide minerals (activated for 0, 30, 60 min), corresponding to the values of 524.659, 518.858 and 513.3 °C, respectively.
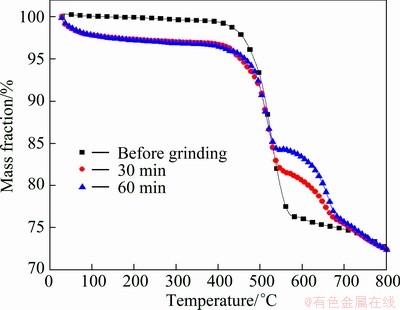
Fig. 6 TG curves of sample 2 before and after grinding for different time

Fig. 7 DSC curves of sample 2 before and after grinding for different time
Table 2 shows some peak temperatures on DSC curves for sample 2. It can be seen that the initial temperature of heat release and the second peak temperature decrease gradually with increasing the milling time. These observations may be caused by the loss of crystallinity, or the dehydration effect by mechanochemistry [22,23]. In Refs. [24-26], the activation energies of activated sulfide minerals decrease with increasing grinding time. Therefore, it can be concluded that the reaction activity of mechanically activated sulfide minerals is higher than that of the non-activated ores; the activated samples are more liable to be oxidized under the same environmental condition.
In a word, the comminution of sulfide minerals in the mining is mainly completed by mechanical device, blasting shock wave, and ground pressure. There are all kinds of modes for the force exertion, and the broken degree of sulfide ores in stope depends on the joint actions of diverse forces. Accordingly, the physicochemical changes are induced in sulfide minerals, such as new surfaces formation, crystal defect, lattice distortion, and decreased reaction temperature, etc; therefore, the chemical reactivity of sulfide minerals is further enhanced. In proper environment, sulfide minerals will experience oxidizing and self-heating to result in spontaneous combustion finally. The whole process can be depicted as Fig. 9.

Fig. 8 DTG curves of sample 2 before and after grinding for different time
Table 2 Peak temperatures on DSC curves for sample 2 before and after mechanical activation
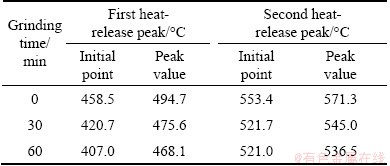
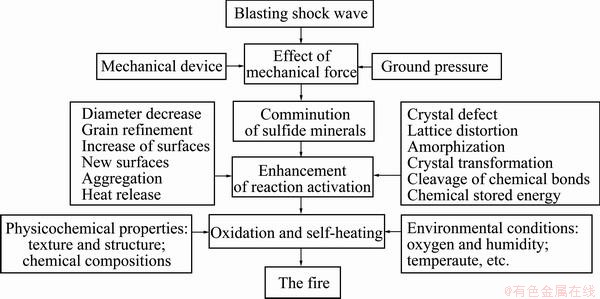
Fig. 9 Mechanical activation mechanism for spontaneous combustion of sulfide minerals
4 Conclusions
1) The samples after the mechanical activation exhibit many pronounced changes with aggregation, decreased particle sizes, increased specific surface areas, decreased diffraction peak intensity, broadened diffraction peak, declined initial temperatures of heat release, and declined auto-ignition points. These observations bring about the formation of active surfaces and lead to the increase in the chemical reactivity of sulfide minerals.
2) The mechanism for spontaneous combustion of sulfide minerals can be explained by the mechanical activation theory. The reaction activation of sulfide minerals is enhanced by all kinds of mechanical forces during the mining, which refer to the general pressure, the friction force, the forces generated by impact crushing and explosion shock wave, etc. Therefore, the oxidation and self-heating reactions take place more easily, and spontaneous combustion occurs ultimately in the proper environment.
References
[1] GU De-sheng, LI Xi-bing. Modern mining science and technology for metal mineral resources [M]. Beijing: Metallurgical Industry Press, 2006: 311-355. (in Chinese)
[2] WU Chao, LI Zi-jun, YANG Fu-qiang, HU Han-hua, GU De-sheng. Risk forecast of spontaneous combustion of sulfide ore dump in a stope and controlling approaches of the fire [J]. Archives of Mining Science,2008, 53(4): 565-579.
[3] YANG Fu-qiang, WU Chao, HU Han-hua, LI Zi-jun, LIU Hui. Fire-extinguishing techniques research on spontaneous combustion of a sulfide iron ore dump in mining stope [C]// LI Sheng-cai, WANG Ya-jun, HUANG Ping. Progress in Safety Science and Technology. Beijing: Science Press, 2008: 869-874.
[4] YANG Fu-qiang, WU Chao, WU Guo-min, LI Yan-qiang, HUANG Xiao-mei. Prediction and forecast techniques of spontaneous combustion of sulfide ore piles [J]. China Safety Science Journal, 2007, 17(5): 89-95. (in Chinese)
[5] LI Zi-jun. Key technique research on spontaneous combustion mechanism and prevention of sulfide ores [D]. Changsha: School of Resources and Safety Engineering, Central South University, 2007: 58-94. (in Chinese)
[6] WANG H H, DLUGOGORSKI B Z, KENNEDY E M. Coal oxidation at low temperatures: Oxygen consumption, oxidation products, reaction mechanism and kinetic modeling [J]. Progress in Energy and Combustion Science, 2003, 29: 487-513.
[7] WU Chao, MENG Ting-rang. Experimental investigation on chemical thermodynamics behavior of sulphide ores during spontaneous combustion [J]. West-China Exploration Engineering, 1995, 7: 57-65.
[8] QIU Yong-hai, CHEN Bai-zhen. Electrochemical mechanism of spontaneous combustion of metal sulfide ores [J]. The Chinese Journal of Nonferrous Metals, 1995, 5(4): 1-4. (in Chinese)
[9] YANG Song-rong, QIU Guan-zhou, HU Yue-hua. Discussion on sulfides bio-oxidation mechanism [J]. Nonferrous Metals, 2003, 55(13): 80-83. (in Chinese)
[10] YANG Fu-qiang, WU Chao, LI Zi-jun. Investigation of the propensity of sulfide concentrates to spontaneous combustion in storage [J]. Journal of Loss Prevention in the Process Industries, 2011, 24: 131-137.
[11] WARRIS C J, MCCORMICK P G. Mechanochemical processing of refractory pyrite [J]. Minerals Engineering, 1997, l0(10): 1119-1125.
[12] BALAZ P. Mechanical activation in hydrometallurgy [J]. Int J Miner Process, 2003, 72: 341-354.
[13] BALAZ P, TAKACS L, LUXOVA M, GODOCIKOVA E, FICERIOVA J. Mechanochemical processing of sulphidic minerals [J]. Int J Miner Process, 2004, 74(S): s365-s371.
[14] GODOCKOVA E, BALAZ P, BASTL Z, BRABEC L. Spectroscopic study of the surface oxidation of mechanically activated sulphides [J]. Applied Surface Science, 2002, 200: 36-47.
[15] HU Hui-ping, CHEN Qi-yuan, YIN Zhou-lan, HE Yue-hui, HUANG Bai-yun. Mechanism of mechanical activation for sulfide ores [J]. Transactions of Nonferrous Metals Society of China, 2007, 17: 205-213.
[16] POURGHAHRAMANI P, FORSSBERG E. Effects of mechanical activation on the reduction behavior of hematite concentrate [J]. Int J Miner Process, 2007, 82: 96-105.
[17] POURGHAHRAMANI P, FORSSBERG E. Comparative study of microstructural characteristics and stored energy of mechanically activated hematite in different grinding environments [J]. Int J Miner Process, 2006, 79: 120-139.
[18] POURGHAHRAMANI P, FORSSBERG E. Microstructure characterization of mechanically activated hematite using XRD line broadening [J]. International Journal of Mineral Processing, 2006, 79: 106-109.
[19] EYMERY J, YLLI F. Study of a mechanochemical transformation in iron pyrite [J]. Journal of Alloys and Compounds, 2000, 298: 306-309.
[20] PALANIANRY S, AZIZI K A M, HUSSIN H, HASHIM S F S. Study on mechanochemical effect of silica for short grinding period [J]. Int J Miner Process, 2007, 82: 195-202.
[21] JONES G C, CORIN K C, van HILLE R P, HARRISON S T L. The generation of toxic reactive oxygen species (ROS) from mechanically activated sulphide concentrates and its effect on thermophilic bioleaching [J]. Minerals Engineering, 2011, 24: 1198-1208.
[22] HU Hui-ping, CHEN Qi-yuan, YIN Zhou-lan, ZHANG Ping-min. Thermal behaviour of mechanically activated pyrites by thermogravimetry(TG) [J]. Thermochimica Acta, 2002, 398: 233-240.
[23] WU Qi-sheng. Mechanicochemistry of inorganic materials [M]. Beijing: Chemical Industry Press, 2008. (in Chinese)
[24] ZHAO Zhong-wei, ZHANG You-xin, CHEN Xing-yu, CHEN Ai-liang, HUO Guang-sheng. Effect of mechanical activation on the leaching kinetics of pyrrhotite [J]. Hydrometallurgy, 2009, 99: 105-108.
[25] HU Hui-ping, CHEN Qi-yuan, YIN Zhou-lan, ZHANG Ping-min, ZOU Jian-peng, CHE Hong-sheng. Study on the kinetics of thermal decomposition of mechanically activated pyrite [J]. Thermochimica Acta, 2002, 389: 79-83.
[26] XIAO Zhong-liang, CHEN Qi-yuan, YIN Zhou-lan, HU Hui-ping, WU Dao-xin. Calorimetric investigation on mechanically activated storage energy mechanism of sphalerite and pyrite [J]. Thermochimica Acta, 2005, 436: 10-14.
硫化矿自燃的机械活化机理
阳富强1,2,吴 超2
1. 福州大学 环境与资源学院,福州 350108;
2. 中南大学 资源与安全工程学院,长沙 410083
摘 要:为了揭示硫化矿发生自燃的本质原因,从典型金属矿山采集了具有代表性的矿样,在室内开展了硫化矿的机械活化实验。采用扫描电镜(SEM)、X射线衍射技术(XRD)、综合热分析技术(STA)表征了矿样在经历不同时间的机械活化作用后的表观形貌、微观结构和热行为等物理化学性质的差异。结果发现:硫化矿石经历机械活化作用后,比表面积增大,出现团聚效应,产生晶格畸变与晶格缺陷,初始放热点及最大反应速率所对应的温度值均有所下降。由此提出了一种新的解释硫化矿自燃的机械活化理论,认为在金属矿山开采中施加于矿体上的各种机械力使得硫化矿产生了机械活化效应,从而使化学反应活性得到相应提高,在一定的环境条件下更加容易产生氧化自热,最终引发自燃火灾。
关键词:金属矿山;采矿;硫化矿;自燃;机械活化;反应机理;化学反应活性
(Edited by Hua YANG)
Foundation item: Project (2012J05088) supported by the Natural Science Foundation of Fujian Province, China; Project (022409) supported by the School Talent Foundation of Fuzhou University, China
Corresponding author: Fu-qiang YANG; Tel: +86-591-22866082; E-mail: ouyangfq@163.com
DOI: 10.1016/S1003-6326(13)62457-7
Abstract: In order to uncover the intrinsic reasons for spontaneous combustion of sulfide minerals, representative samples were collected from typical metal mines to carry out the mechanical activation experiment. The structures and heat behaviors of activated samples were characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD) analysis, and simultaneous thermal analysis (STA). It is found that the sulfide minerals after mechanical activation show many changes with increased specific surface areas, aggregation phenomenon, decreased diffraction peak intensity, broadened diffraction peak, declined initial temperatures of heat release and self-ignition points. A new theory for explaining the spontaneous combustion of sulfide minerals is put forward: the chemical reaction activity of sulfide minerals is heightened by all kinds of mechanical forces during the mining, and the spontaneous combustion takes place finally under proper environment.


