
J. Cent. South Univ. (2020) 27: 1654-1665
DOI: https://doi.org/10.1007/s11771-020-4397-8
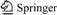
Microstructure and corrosion behavior of as-cast ADC12 alloy with rare earth Yb addition and hot extrusion
HE Jia-jia(贺佳佳)1, 2, YAN Hong(闫洪)1, 2, ZOU Yong-cheng(邹永成)1, 2,YU Bao-biao(喻保标)1, 2, HU Zhi(胡志)1, 2
1. School of Mechanical and Electrical Engineering, Nanchang University, Nanchang 330031, China;
2. Key Laboratory of Light Alloy Preparation and Processing in Nanchang City, Nanchang 330031, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract:
The effects of rare earth ytterbium (Yb) addition and hot extrusion on the microstructure and corrosion behavior of as-cast ADC12 were studied by optical microscopy (OM), scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS) and X-ray diffraction (XRD). The experimental results demonstrate that both the Si phase and β-Al5FeSi phase in the alloy with 0.9 wt% Yb have been remarkably refined, and the Al3Yb intermetallic compound has also been obtained. The Si, β-Al5FeSi, and rare earth phases are further refined in the alloy at 0.9 wt% Yb and hot extrusion. The results of the immersion corrosion tests and electrochemical experiments show that the corrosion current density (8.56 μA/cm2) of the alloy with 0.9 wt% Yb addition and hot extrusion is 50.6% lower than the untreated alloy (17.33 μA/cm2), and the polarization resistance (9252 Ω·cm2) was 71.3% higher than the untreated alloy (2654 Ω·cm2). The corrosion in the cathode phase in the micro-battery was refined to varying degrees attributable to the addition of Yb and hot extrusion, where the cathode reaction in the corrosion process caused a decrease of the corrosion rate.
Key words:
ADC12 alloy; rare earth Yb addition; hot extrusion; microstructure; corrosion resistance;
Cite this article as:
HE Jia-jia, YAN Hong, ZOU Yong-cheng, YU Bao-biao, HU Zhi. Microstructure and corrosion behavior of as-cast ADC12 alloy with rare earth Yb addition and hot extrusion [J]. Journal of Central South University, 2020, 27(6): 1654-1665.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-020-4397-81 Introduction
Aluminum alloys are widely used in the aerospace and transportation fields because of their low density, high specific strength, good electrical and thermal conductivities, and good plastic forming performance [1-4]. In particularly, it has been widely used in automobile gearboxes, crankcases, electrical equipment components, industrial machinery components, optical equipment components, and household appliances [5, 6]. ADC12 alloy belongs to the Al-Si-Cu alloy and the die-cast aluminum alloy. One of the most important properties of ADC12 is that it has good mechanical properties in the as-cast state. However, the chemical composition and casting conditions of the alloy are not controlled properly, and the surface corrosion resistance of the casting is poor [7-9]. Furthermore, the mechanical properties are greatly reduced due to the corrosion of the alloy [10].
Therefore, in order to meet the performance requirements in various environments, researchers have performed different surface treatments on aluminum alloys for different purposes [11-13]. Currently, many studies have been performed to improve the mechanical properties of aluminum alloys, such as rare earth modification and hot extrusion. LI et al [14] investigated the addition of cerium to refine the primary Si phase, thereby increasing the ultimate tensile strength and elongation of the Al-20% Si alloy. JOY-YII et al [15] found that the addition of rare earth elements to Al-Si alloys can refine the grain size of primary Si, increase the tensile strength and reduce the friction coefficient. TZENG et al [16] studied the effects of the addition of Sc on the microstructure and mechanical properties of Al-11.6Si alloys. The results indicated that the addition of Sc can form the Al3Sc precipitates, inhibit the growth of crystal grains, and improve ductility. Hot extrusion, a conventional thermo-mechanical process widely used in wrought aluminum alloys, is an effective method to refine grains and reduce casting defects. Many studies [17-19] have found that the hot extrusion can significantly improve the mechanical properties of the alloy, especially the plasticity. DING et al [20] found that Al-12.0%Si-0.2%Mg alloy after aged for 12 h by hot extrusion resulted in a good combination of tensile strength and elongation of 256.3 MPa and 15%, respectively. LIANG et al [21] studied the Si and Al3FeSi particles in Al-7Si-0.3Mg alloy broken and redistributed along the extrusion direction during the extrusion process, so that the elongation at break of the alloy reached 16.6%-20.5%. The rare earth metamorphism and hot extrusion can improve the microstructure and mechanical properties of the alloy, while the cost of Al alloy notably increases with the addition of rare earth elements, significantly restricting their commercial application in the electronics and automotive industries. Therefore, researchers have also developed some RE-low alloys combining hot extrusion and achieved obvious effects. WEI et al [22] determined that the addition of Y and the hot extrusion process clearly improved the microstructural and mechanical properties. HAMIDREZA et al [23] found that extreme tensile strength and elongation of the Al-15wt%Mg2Si could be increased to 245.83 MPa and 7.65%, respectively, with the addition of 1% Ga and hot extrusion. From these studies, it can be summarized that both rare earth element addition and hot extrusion can improve the microstructural and mechanical properties of Al-Si alloys.
Hence, many investigations have indicated that the addition of proper rare earth elements can significantly increase the corrosion resistance of the Al-Si alloy. ARRABAL et al [24] found that the microstructure of A356 can be refined by adding rare earth Nd and Nd-rich phases leading to the improvement of the pitting corrosion resistance of A356. ZOU et al [25] found that adding varying contents of rare earth metal Yb to the ADC12 alloy can effectively refine the grain size and enhance the corrosion resistance. These properties were optimized when 0.9 wt% of rare earth element was incorporated. At present, there are some researches on the corrosion resistance of Al-Si alloy with the addition of rare earth, but there are few researches on the corrosion resistance of Al-Si alloy with the subsequent processing after the addition of rare earth. Based on these observations, the current study aimed to study the effects of rare earth Yb (0.9 wt%) addition and hot extrusion on the microstructural and corrosion behavior of as-cast ADC12 alloy.
2 Experiment
2.1 Preparation of experimental materials
The materials studied in this experiment were as-cast ADC12 and rare earth Yb. In this experiment, rare earth Yb was added to ADC12 by applying high-energy ultrasonic vibration. The ADC12 alloy was melted in a graphite crucible with a resistance furnace heated to 750 °C. After the ADC12 was completely melted, an Al-10Yb master alloy was added to the molten alloy and mechanically stirred. The molten alloy was maintained at 750 °C and ultrasonically vibrated for 5 min. After the vibration step, gases and impurities were removed from the molten alloy. Then the aluminum liquid was quickly tipped into a preheated 200 °C mold (15 mm in diameter and 15 cm in height). The chemical composition of ADC12 and ADC12 + 0.9 wt% Yb was measured by ICP-AES (inductively coupled plasma-atomic emission spectrometry), and the results are shown in Table 1. The hot extrusion machine was preheated to a temperature of 400-420 °C, and then the samples were carried out preheating accompany with the extruder warming up for 4 h. The extrusion ratio was 8:1. The sample rod was extruded at an extrusion speed of 10 mm/min, and the hot extruded sample was quenched at 60-100 °C to retain the deformed structure immediately after hot extrusion ADC12 + hot extrusion, and ADC12 + 0.9 wt% Yb+ hot extrusion.
Table 1 Chemical composition of as-cast ADC12 and ADC12 + 0.9 wt% Yb
2.2 Material preparation and microstructure
The center positions of the cylindrical samples were cut longitudinally into five sets of circular samples, and the same sizes were d15 mm×2 mm. A set of samples were ground and polished, and then etched with hydrofluoric acid (0.5 wt%). The microstructural changes of each sample were observed by optical microscope (OM, Nican M300 microscope). The phases of the sample were further analyzed by scanning electron microscopy (SEM NOVA NANOSEM 450), electronic differential system (EDS INCA 250X-Max 50), and X-ray diffraction (XRD D8 ADVANCE).
2.3 Immersion corrosion test
Before the experiment, the samples were ground on SiC sandpaper to a uniform roughness and then immersed in a 95% ethanol solution for 5 min. Then, the samples were placed vertically in beakers containing 3.5 wt% NaCl etching solution (pH=7), and the beakers were placed in a thermostatic water bath kettle which had been set at 35 °C for 40 d. The samples were first washed with hydrofluoric acid, and then placed in a 95% ethanol solution for ultrasonic cleaning to remove the residual NaCl particles and corrosion products.
2.4 Electrochemical measurement
Three groups of samples were taken for electrochemical measurement using the Princeton P4000 electrochemical workstation. The samples were ground and polished, washed with alcohol and distilled water, and then dried for testing. The electrochemical test was carried out in a three-electrode electrolytic cell. The tested sample was used as a working electrode (effective exposure area was 1 cm2). The platinum plate electrode acts as the opposite electrode and a saturated calomel electrode as a reference electrode. The electrolyte was 3.5 wt% NaCl solution. All the electrochemical tests were performed at room temperature, and the prepared samples were soaked in a 3.5 wt% NaCl solution for 24 h before the electrochemical experiments and then ultrasonically cleaned and tested. The polarization curves were obtained at a scan speed of 2 mV/s over a range of ±300 mV. The electrochemical impedance spectroscopy (EIS) test was performed over a frequency sweep range from 0.1 to 106 Hz with a sinusoidal alternating current signal of 10 mV at amplitude. The Tafel polarization curve and EIS were analyzed by Versa Stiduo software and Zview, respectively. To confirm statistical integrity, all electrochemical measurements were repeated five times.
3 Analysis of experimental results
3.1 Microstructure
The microstructures of the samples are shown in Figure 1. As shown in Figure 1(a), it can be seen that the α-Al phase of the untreated as-cast alloy is not obvious, and the Si phase is rough, long needle-like, and disorderly distributed with many distributed, coarse bulk β-Al5FeSi phases. As shown in Figure 1(b), it is the microstructure of the hot extruded ADC12 alloy. It can be seen that most Si phases are spherical, and a few are short rod-shaped, with a spherification rate slightly lower than that of the alloy added with rare earth and extruded. The β-Al5FeSi phase was squeezed into tiny clumps or short bars. In Figure 1(c), the α-Al phase of ADC12+0.9 wt% Yb begins to show a clear outline and has an elliptical shape, and the Si phase is transformed from the long needle-like into a fine fibrous or granular shape, and the β-Al5FeSi phase becomes a fine block. In Figure 1(d), the microstructure of ADC12 + 0.9 wt% Yb + hot extrusion shows that the fine fibrous eutectic Si phase was extruded into a finer granular Si phase and distributed along the extrusion direction. The β-Al5FeSi phase is extruded into a short rod shape, a finer block, or granule, and the α-Al phase is elongated along the extrusion direction. The α-Al phase adjacent to the extrusion direction becomes connected, and the secondary dendrite arm distance is further reduced compared to that without hot extrusion.

Figure 1 Microstructures of as-cast ADC12 (a), ADC12+hot extrusion (b), ADC12+0.9 wt% Yb (c), and ADC12+0.9 wt% Yb + hot extrusion (d)
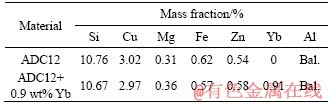
The SEM micrographs of ADC12 + 0.9 wt% Yb and ADC12 + 0.9 wt% Yb + hot extrusion are shown in Figure 2. In Figure 2(a), the bright white long needle-like phase is probably the Yb-rich intermetallic compound. To further confirm the composition of the phase, the EDS of the bright white phase is shown in Figure 2 and the XRD analysis of ADC12 + 0.9 wt% Yb is shown in Figure 3. Compared with the XRD patterns of the as-cast ADC12 alloy and the rare earth Yb modified alloy, the change of peak quantity and peak value is very significant. It is confirmed that the rare earth intermetallic compound in the alloy is Al3Yb, and the α-Al, Si, and β-Al5FeSi phases are also found. Therefore, as shown in Figure 2(b), it can be observed that the rare earth phase after hot extrusion is changed from the original long needle-like to the short rod shape, and the distribution is more uniform.
3.2 Corrosion behavior
3.2.1 Characterization of corrosion surfaces
The corrosion morphologies of alloys after 40 d of corrosion in 3.5% NaCl solution are shown in Figure 4. It can be seen from Figure 4(a) that the untreated as-cast alloy has a relatively large corrosion hole, accompanied by a large area of pitting corrosion. The alloy with 0.9 wt% Yb addition has less pitting corrosion compared to the untreated alloy, and the depth of corrosion hole is decreased. After hot extrusion, there are no obvious corrosion holes, but a spalling corrosion layer is formed locally. Figure 4(b) clearly shows the corrosion morphology of as-cast ADC12 after hot extrusion. There are relatively larger corrosion cavities than the alloy with 0.9 wt%Yb, but smaller than the as-cast ADC12 alloy. The depth of the corrosion holes is deeper than the alloy with 0.9 wt%Yb, but it is shallower than the as-cast ADC12 alloy. Hence, the corrosion resistance of the alloy containing 0.9 wt% Yb via hot extrusion shows the best performance, and the surface is flatter compared to the two other samples.
3.2.2 Tafel polarization curves
Tafel plots were generated for the as-cast ADC12, ADC12 + hot extrusion, ADC12+0.9 wt% Yb, and ADC12 + 0.9 wt% Yb + hot extrusion specimens, as shown in Figure 5. All curves present a similar form, in spite of the different treatment in samples. In addition, it can be seen that the cathode and anode branches of the polarization curves of ADC12 + hot extrusion, ADC12 + 0.9 wt% Yb and ADC12 + 0.9 wt% Yb + hot extrusion undergo corresponding changes. For the anode branch, the anodic polarization curves of ADC12 + hot extrusion, ADC12 + 0.9 wt% Yb and ADC12 + 0.9 wt% Yb + hot extrusion both move toward the noble direction, where the last one is the highest, indicating that the anode dissolution is suppressed. For the cathode branch, the cathodic polarization curves of ADC12 + hot extrusion, ADC12 + 0.9 wt% Yb and ADC12 + 0.9 wt% Yb + hot extrusion moved to the left. The cathodic polarization curve of ADC12 + 0.9 wt% Yb + hot extrusion sample is at the leftmost end, indicating that hydrogen reduction is suppressed.
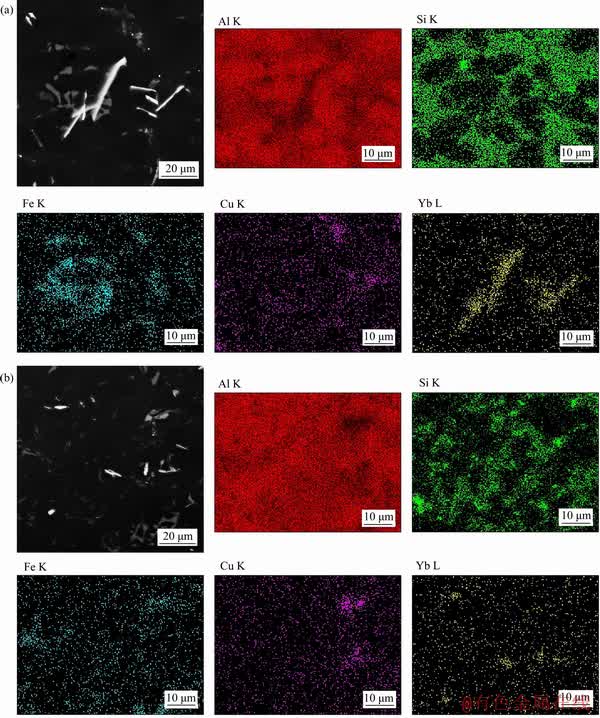
Figure 2 SEM and EDS analysis of ADC12+0.9 wt%Yb (a) and ADC12+0.9 wt%Yb + hot extrusion (b)
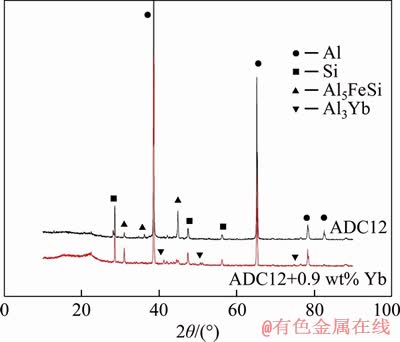
Figure 3 XRD patterns of as-cast ADC12 and ADC12+0.9 wt% Yb
According to the results in Table 2, the corrosion potential of the untreated alloy is in the most negative direction (-0.568 V vs SCE), and the corrosion potential is reversed in the noble direction compared to ADC12 + hot extrusion, ADC12 + 0.9 wt% Yb and ADC12 + 0.9 wt% Yb + hot extrusion samples. The corrosion potential of ADC12 + 0.9 wt% Yb + hot extrusion reaches -0.519 V vs SCE. The values of corrosion current density obtained by the Tafel curve are quite different. The corrosion current density of the untreated as-cast alloy was the largest (17.33 μA/cm2), the ADC12 + hot extrusion (14.32 μA/cm2) was 17.1% lower than the untreated alloy, and the corrosion current density of ADC12 + 0.9 wt% Yb (11.28 μA/cm2) was 34.9% lower than the untreated alloy. The ADC12 + 0.9 wt% Yb + hot extrusion sample showed the lowest corrosion current density (8.56 μA/cm2), which was 50.6% lower than the untreated alloy. The smaller the corrosion current density, the better the corrosion resistance of the alloy, which indicates that the corrosion resistance of the alloy after modification and hot extrusion is the best. It can be seen from the above corrosion current density that both rare earth and hot extrusion can improve the corrosion resistance of the ADC12 alloy, and the corrosion performance of the alloy is the best after the combination of the two.
3.2.3 Electrochemical impedance spectroscopy (EIS)
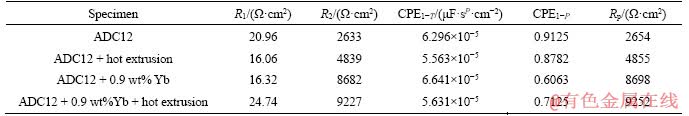
Electrochemical impedance spectroscopy (EIS) was performed on three alloy samples to analyze the corrosion kinetics occurring at the metal/solution interface. Figure 6(a) shows the Nyquist plot of as-cast ADC12, ADC12 + hot extrusion, ADC12 + 0.9 wt% Yb, and ADC12 + 0.9 wt% Yb + hot extrusion samples. The Nyquist curve consists of a capacitor loop, and the diameter of the capacitor circuit after rare earth Yb modification and hot extrusion is larger than that of as-cast ADC12. To analyze the corrosion behavior of the specimens, the equivalent circuit diagram was simulated according to the impedance values obtained from electrochemical experiments (Figure 6(c)). In the loop circuit, R1 represents the solution resistance, and R2 represents the charge transfer resistance. Furthermore, CPE represents the capacitance of the electric double layer at the interface between the metal surface and the corrosive medium. In addition, the value of the polarization resistance (Rp) can be derived as [26]:
Rp=R1+R2 (1)
The calculated values are shown in Table 3. From Table 3, it can be concluded that compared with ADC12 + hot extrusion, ADC12 + 0.9 wt% Yb and ADC12 + 0.9 wt% Yb + hot extrusion samples, the polarization resistance of the as-cast ADC12 is the smallest (2654 Ω·cm2), while the polarization resistance of ADC12 + 0.9 wt% Yb + hot extrusion is the largest (9252 Ω·cm2). The impedance of ADC12 + hot extrusion is higher than the as-cast alloy but much smaller than the ADC12 + 0.9 wt% Yb +hot extrusion. The value of the polarization resistance Rp is a very important parameter in evaluating the corrosion resistance of the alloy. The larger the Rp, the better the corrosion resistance of the alloy [27, 28], which means that corrosion resistance of the alloy after metamorphism and hot extrusion is the best. This is consistent with the results of the previously fitted corrosion current.
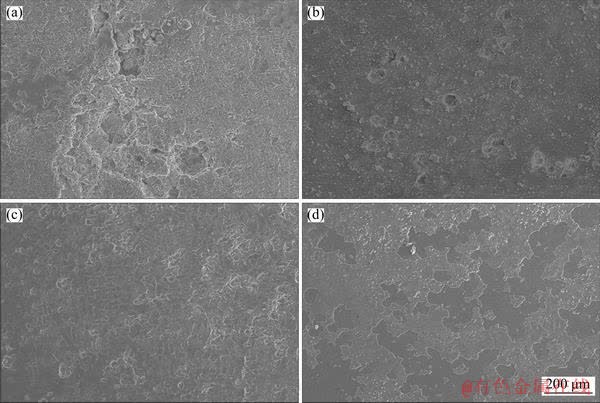
Figure 4 Corrosion morphology of as-cast ADC12 (a), ADC12 + hot extrusion (b), ADC12 + 0.9 wt% Yb (c), and ADC12 + 0.9 wt% Yb + hot extrusion (d) immersed in 3.5 wt% NaCl solution for 40 d
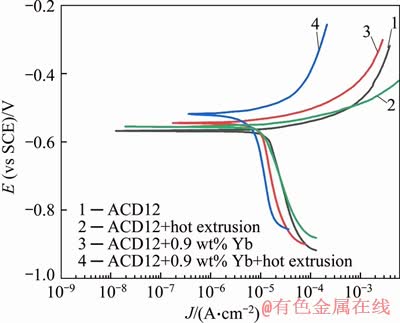
Figure 5 Tafel polarization curves of ADC12 alloys
Table 2 Ecorr, Jcorr, cathodic Beta and anodic Beta of ADC12 alloys
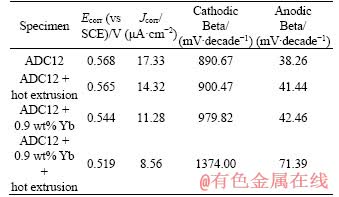
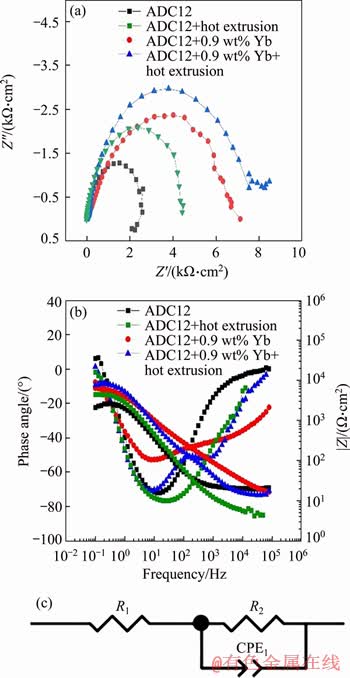
Figure 6 Nyquist plot (a), Bode plot (b), and equivalent circuit (c) of ADC12 alloys
The Bode plot shows the change of impedance ( Z
Z ) and phase angle of all samples with respect to frequency for all the specimens, as shown in Figure 6(b). The figure shows the impedance values of the three frequency regions of low, intermediate and high frequency. The high frequency region indicates the characteristics of the reference electrode and solution resistance, the intermediate frequency region represents the surface capacitance behavior, and the low frequency region represents the charge transfer process at the interface of the metal solution. It can be seen from the figure that in the low frequency region, the impedance value of the as-cast ADC12 is the smallest, followed by ADC12 + hot extrusion, ADC12 + 0.9 wt% Yb, and ADC12 + 0.9 wt% Yb + hot extrusion has the highest impedance in the low frequency region, which is consistent with the results exhibited by the Nyquist plot.
) and phase angle of all samples with respect to frequency for all the specimens, as shown in Figure 6(b). The figure shows the impedance values of the three frequency regions of low, intermediate and high frequency. The high frequency region indicates the characteristics of the reference electrode and solution resistance, the intermediate frequency region represents the surface capacitance behavior, and the low frequency region represents the charge transfer process at the interface of the metal solution. It can be seen from the figure that in the low frequency region, the impedance value of the as-cast ADC12 is the smallest, followed by ADC12 + hot extrusion, ADC12 + 0.9 wt% Yb, and ADC12 + 0.9 wt% Yb + hot extrusion has the highest impedance in the low frequency region, which is consistent with the results exhibited by the Nyquist plot.
Table 3 Electrochemical parameters of studied alloys attained from EIS data
4 Discussion
According to Mullins-Sekerka’s theory of interface stability kinetics, the critical conditions for maintaining interface stability are given as [29, 30]:
GS∕v≥[mLC0(1-k)/(kDL)]·[(λS+λL)/(2λL)]·Φ+
ρLH/(2λL) (2)
where k can be expressed as:
k=CS/CL (3)
In Eq. (2), GS represents the temperature gradient in the solid phase; v represents the crystal growth rate; mL represents the slope of a liquidus in the phase diagram; C0 represents the original composition concentration of the alloy; k represents the solute partition coefficient; DL represents liquid phase solute diffusion coefficient; λS and λL represent the thermal conductivities of the solid phase and the liquid phase, respectively; ρL represents the liquid phase density; H represents the latent heat of crystallization of melt per unit volume; Φ represents a dimensionless parameter; CS and CL represent the equilibrium solubilities of the solid phase and the liquid phase, respectively. When 0.9 wt% of rare earth Yb is added, due to the low solid solubility of rare earth Yb in the alloy matrix, it will accumulate at the front of the solid-liquid interface during solidification. It also hinders the dissolution of the solute, resulting in a decrease in the value of CS. This means that the value of k decreases, leading to an increase in GS/v, resulting in an increase in the supercooling of the component [31]. In addition, the supercooling value of the component exceeds the degree of supercooling required for the heterogeneous nucleation, so that the melt in the interval undergoes a new nucleation process and the α-Al exhibits an elliptical shape. In this process, the aggregation of the rare earth element Yb hinders the diffusion of other solute elements and refines Si and β-Al5FeSi phases [32].
The main phases in ADC12 alloy are α-Al phase, Si and β-Al5FeSi phases. With the ADC12 alloy in the corrosion process, α-Al reacts as an anode with dissolution, and Si and β-Al5FeSi phases in the alloy react as the cathode phase. The main cathode of Si phase and the adjacent main anode of α-Al matrix form a galvanic cell resulting in the cathodic dissolution. At the beginning, the α-Al matrix of the alloy partially dissolves around the Si phase, and when dissolved to a certain extent, the corrosion holes are formed, as shown in Figure 3(a). After adding rare earth Yb, it can be seen from Figure 1 that the grain size of the Si and the β-Al5FeSi phases is significantly smaller. The rare earth Yb and the Al element form a rare earth phase Al3Yb, which leads to a less active cathodic reaction due to the refinement of the cathode phase. Thus, the corrosion resistance of the alloy improved. After hot extrusion, the Si, β-Al5FeSi and rare earth phases are rearranged along the extrusion direction and the size reduces. Thereby, the cathode reaction was further suppressed and the corrosion resistance of the alloy was improved.
To further study the corrosion process of the as-cast ADC12 alloy, ADC12 + 0.9 wt% Yb, ADC12 + 0.9 wt% Yb + hot extrusion, a graphical process of the corrosion mechanism is given in Figure 7. In Figure 7(a), two cathode micro- components, the Si and β-Al5FeSi phases, are found in the micro-battery. When the alloy is immersed in the 3.5 wt% NaCl solution, hydrogen evolution occurs in the cathode phase, and the α-Al phase in the vicinity of the cathode phase is dissolved, leading to electrochemical corrosion. Micro- galvanic corrosion obviously occurred around the main cathode phase of the Si and the β-Al5FeSi phases. Moreover, the corrosion extends to the inside of the alloy along the coarse Si phase and the blocky β-Al5FeSi phase, as shown in Figures 7(a) and (b). As the corrosion process continues, the dissolution of α-Al phase will lead to the formation of large corrosion pits as shown in Figure 3(a). It can be seen from Figure 3(d) that the Si and β-Al5FeSi phase of the as-cast ADC12 alloy after hot extrusion are remarkably refined. Therefore, as shown in Figures 7(c) and (d), the cathode phase in the micro-galvanic corrosion cell was refined, the corrosion holes are correspondingly reduced and become smaller, and the corrosion resistance is improved.
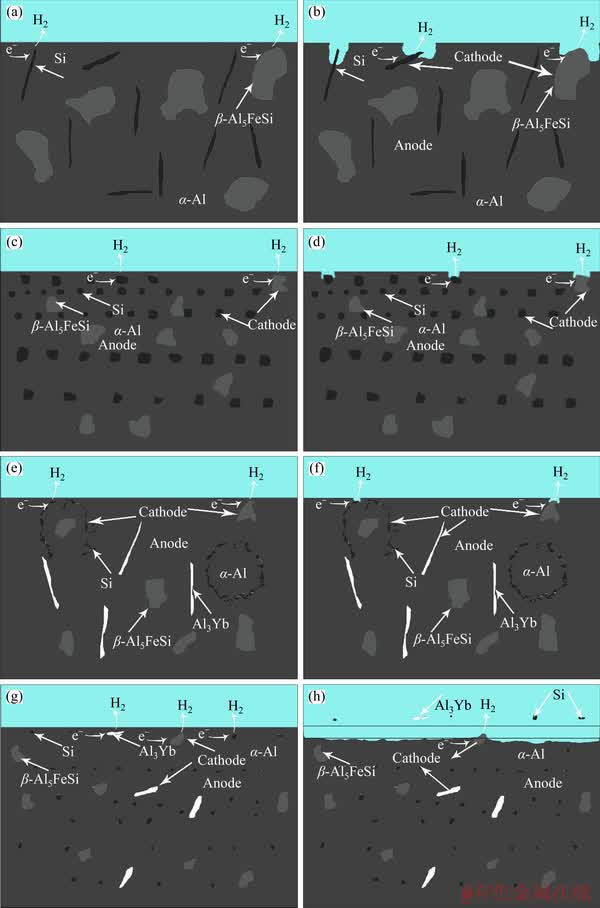
Figure 7 Schematic illustration of as-cast ADC12 (a, b), ADC12 + hot extrusion (c, d), ADC12 + 0.9 wt%Yb (e, f) and ADC12 + 0.9 wt%Yb + hot extrusion (g, h) during corrosion processing
There are three kinds of micro-components in the corrosion micro-battery after adding rare earth Yb. One is Si phase, and the other two are β-Al5FeSi phase and rare earth phase Al3Yb, as shown in Figures 7(e) and (f). The refined Si phase, β-Al5FeSi phase and rare earth phase act as the cathode phase in the corrosion micro-battery, and the corrosion pits become smaller due to the refinement of the cathode phase, as shown in Figure 3(b). After hot extrusion, the Si, β-Al5FeSi, and rare earth phases are further refined and uniformly distributed. It can be seen that the small and uniformly distributed cathode phase provides extra corrosion sites for pitting corrosion as shown in Figures 7(g) and (h), resulting in an increased number of corrosion pits. As the pitting corrosion hole expands, the β-Al5FeSi and the rare earth phases on the alloy peel off. The shedding of these two phases prevents corrosion from continuing inside the alloy and inhibits further corrosion. Therefore, local exfoliation corrosion is formed in the alloy with 0.9 wt% incorporated Yb and hot extrusion as shown in Figure 3(c). The above observations show that the refinement of the cathode phase can improve the corrosion resistance of the ADC12 alloy. It is inferred that the corrosion resistance of the alloy is related to the distribution and the size of the grains of the alloy.
5 Conclusions
This study primarily researches the effects of rare earth Yb addition and hot extrusion on the microstructure and corrosion behavior of as-cast ADC12, and the following results are obtained.
1) The addition of the rare earth Yb can effectively refine the Si phase and β-phase of the as-cast ADC12 alloy. The Si phase is transformed from the original rough and long needle-like to the fibrous shape, and the β-Al5FeSi phase is refined to some extent. The results show that rare earth Yb can refine the Si phase and β-Al5FeSi phase structure of the alloy.
2) The Si phase of the alloy with rare earth element addition and hot extrusion is refined into a granular shape and uniformly distributed along the extrusion direction. The β-Al5FeSi phase is extruded into short rods or granules, and the rare earth element phase is also refined. It indicates that the extrusion can further improve the microstructure of the alloy.
3) After the addition of rare earth element Yb and hot extrusion, the cathodic phase in the micro-battery is refined, thus inhibiting the cathode reduction process in the micro-galvanic corrosion. The corrosion current density of ADC12 + 0.9 wt% Yb + hot extrusion (8.56 μA/cm2) is 50.6% lower than the corrosion current density of untreated ADC12 alloy (17.33 μA/cm2). The highest polarization resistance of ADC12 + 0.9 wt% Yb + hot extrusion (9252 Ω·cm2) sample is 71.3% higher than the untreated alloy (2654 Ω·cm2). These results show that the corrosion resistance of ADC12 + 0.9 wt% Yb + hot extrusion is the best.
References
[1] ZHANG Xue-song, CHEN Yong-jun, HU Jun-ling. Recent advances in the development of aerospace materials [J]. Progress in Aerospace Sciences, 2018, 97: 22-34. DOI: 10.1016/j.paerosci.2018.01.001.
[2] DURSUN T, SOUTIS C. Recent developments in advanced aircraft aluminium alloys [J]. Materials & Design, 2014, 56: 862-871. DOI: 10.1016/j.matdes.2013.12.002.
[3] STARKE E A Jr, STALEY J T. Application of modern aluminum alloys to aircraft [J]. Progress in Aerospace Sciences, 1996, 32(2, 3): 131-172. DOI: 10.1016/0376- 0421(95)00004-6.
[4] YE Hai-zhi. An overview of the development of Al-Si-alloy based material for engine applications [J]. Journal of Materials Engineering and Performance, 2003, 12(3): 288-297. DOI: 10.1361/105994903770343132.
[5] PUDOVIKOV E I. Parts from aluminum alloys (body of the wheelpair reduction gearbox) [J]. Metal Science & Heat Treatment, 2000, 42: 196-200. DOI: 10.1007/ BF02469849.
[6] GAYLE F W, GOODWAY M. Precipitation hardening in the first aerospace aluminum alloy: The wright flyer crankcase [J]. Science, 1994, 266(5187): 1015-1017. DOI: 10.1126/ science.266.5187.1015.
[7] SLATTERY B E, PERRY T, EDRISY A. Microstructural evolution of a eutectic Al–Si engine subjected to severe running conditions [J]. Materials Science and Engineering A, 2009, 512(1, 2): 76-81. DOI: 10.1016/j.msea.2009. 01.025.
[8] MILLER W S, ZHUANG L, BOTTEMA J, WITTEBROOD A J, de SMET P, HASZLER A, VIEREGGE A. Recent development in aluminium alloys for the automotive industry [J]. Materials Science and Engineering A, 2000, 280(1): 37-49. DOI: 10.1016/s0921-5093(99)00653-x.
[9] OKAYASU M, OHKURA Y, TAKEUCHI S, TAKASU S, OHFUJI H, SHIRAISHI T. A study of the mechanical properties of an Al–Si–Cu alloy (ADC12) produced by various casting processes [J]. Materials Science and Engineering A, 2012, 543: 185-192. DOI: 10.1016/j.msea. 2012.02.073.
[10] LIAO Wen-bo, LIU Xin-yu, LIU Sheng-dan, ZHANG Xin-ming. Effect of exfoliation corrosion on mechanical properties of 7055 aluminum alloy sheet [J]. Journal of Central South University, 2012, 43(6): 2137-2141. DOI: 10.1109/INTMAG.2005.1463725.
[11] PAN J, LEE S P, YOSHIDA M, SASAKI G, FUTAMA N, FUJII T, FUKUNAGA H. Effect of whisker surface treatment on the strength of Al18B4O33/Al alloy composites [J]. Advanced Composite Materials, 2001, 10(4): 299-307. DOI: 10.1163/156855101753415328.
[12] YUE T M, YAN L J, CHAN C P, DONG C F, MAN H C, PANG G K H. Excimer laser surface treatment of aluminum alloy AA7075 to improve corrosion resistance [J]. Surface and Coatings Technology, 2004, 179(2, 3): 158-164. DOI: 10.1016/s0257-8972(03)00850-8.
[13] WAGNER L. Mechanical surface treatments on titanium, aluminum and magnesium alloys [J]. Materials Science and Engineering A, 1999, 263(2): 210-216. DOI: 10.1016/s0921- 5093(98)01168-x.
[14] LI Qing-lin, XIA Tian-dong, LAN Ye-feng, ZHAO Wen-jun, FAN Lu, LI Peng-fei. Effect of rare earth cerium addition on the microstructure and tensile properties of hypereutectic Al-20%Si alloy [J]. Journal of Alloys & Compounds, 2013, 562(1): 25-32. DOI: 10.1016/j.jallcom.2013.02.016.
[15] JOY-YII S L, KURNIAWAN D. Effect of rare earth addition on microstructure and mechanical properties of Al-Si alloys: An overview [J]. Advanced Materials Research, 2013, 845: 27-30. DOI: 10.4028/www.scientific.net/AMR.845. 27.
[16] TZENG Y C, JIAN S Y. Effects of the addition of trace amounts of Sc on the microstructure and mechanical properties of Al-11.6Si alloys [J]. Materials Science and Engineering A, 2018, 723: 22-28. DOI: 10.1016/j.msea.2018. 03.016.
[17] JIN P, XIAO B, WANG Q. Effect of hot extrusion on interfacial microstructure and tensile properties of SiCp/2009Al composites fabricated at different hot pressing temperatures [J]. Journal of Materials Science & Technology, 2011, 27(6): 40-46. DOI: 10.1016/S1005-0302(11)60101-1.
[18] HSIANG S H, KUO J L. An investigation on the hot extrusion process of magnesium alloy sheet [J]. Journal of Materials Processing Technology, 2003, 140(1-3): 6-12. DOI: 10.1016/s0924-0136(03)00693-9.
[19] YU J, ZHAO G, CHEN L. Investigation of interface evolution, microstructure and mechanical properties of solid-state bonding seams in hot extrusion process of aluminum alloy profiles [J]. Journal of Materials Processing Technology, 2016, 230: 153-166. DOI: 10.1016/j.jmatprotec. 2015.11.020.
[20] DING Ke, LIAO Heng-cheng, JIN Qiu-min, TANG Yun. Effect of hot extrusion on mechanical properties and microstructure of near eutectic Al–12.0%Si–0.2%Mg alloy [J]. Materials Science and Engineering A, 2010, 527(26): 6887-6892. DOI: 10.1016/j.msea.2010.07. 068.
[21] LIANG J M, GUO X Q, ZHENG Y F. Effect of extrusion temperature on microstructural evolution and intergranule bonding of Al-7Si-0.3Mg (wt%) alloy rods produced by extrusion of granule compacts [J]. Journal of Materials Processing Technology, 2016, 232: 78-89. DOI: 10.1016/ j.jmatprotec.2016.01.030.
[22] WEI Zhi-fan, LEI Yu-shun, YAN Hong, XU Xi-hao, HE Jia-jia. Microstructure and mechanical properties of A356 alloy with Y addition processed by hot extrusion [J]. Journal of Rare Earths, 2019, 37(6): 659-667. DOI: 10.1016/ j.jre.2018. 11.008.
[23] HAMIDREZA G, MOHD H I, NORHAYATI A. Effect of hot extrusion on microstructural evolution and tensile properties of Al-15%Mg2Si-xGd in-situ composites [J]. Journal of Alloys and Compounds, 2018, 751: 370-390. DOI: 10.1016/ j.jallcom.2018.04.131.
[24] ARRABAL R, MINGO B, PARDO A, MOHEDANO M, MATYKINA E, MERINO M C, RIVAS A. Microstructure and corrosion behaviour of A356 aluminium alloy modified with Nd [J]. Materials and Corrosion, 2015, 66(6): 535-541. DOI: 10.1002/maco.201407674.
[25] ZOU Yong-cheng, YAN Hong, YU Bao-biao, HU Zhi. Effect of rare earth Yb on microstructure and corrosion resistance of ADC12 aluminum alloy [J]. Intermetallics, 2019, 110: 106-114. DOI: 10.1016/j.intermet.2019.106487.
[26] YIN Zheng, CHEN Yang, YAN Hong, ZHOU Guo-hua, WU Xiao-quan, HU Zhi. Effects of the second phases on corrosion resistance of AZ91-xGd alloys treated with ultrasonic vibration [J]. Journal of Alloys and Compounds, 2019, 783: 877-885. DOI: 10.1016/j.jallcom.2019. 01.002.
[27] QI Xing, SUN Bin, ZHANG Xiao-yan, QI Wen-juan, WANG Chao, SONG Ren-guo. Effects of cathodic polarization on SCC behavior of AA7003 under various aging treatments [J]. Journal of Central South University, 2018, 25(10): 2299-2308. DOI: 10.1007/s11771-018-3914-5.
[28] NIU Hao-yi, CAO Fang-fang, DENG Kun-kun, NIE Kai-bo. Microstructure and corrosion behavior of the as-extruded Mg–4Zn–2Gd–0.5Ca alloy [J]. Acta Metallurgica Sinica (English Letters), 2020, 33: 362-374. DOI: 10.1007/s40195- 019-00984-2.
[29] HUANG Xin, YAN Hong. Effect of trace La addition on the microstructure and mechanical property of as-cast ADC12 Al-alloy [J]. Journal of Wuhan University of Technology- Mater, Sci. Ed, 2013, 28(1): 202-205. DOI: 10.1007/s11595- 013-0665-x.
[30] QIAN M, CAO P, EASTON M A, MCDONALD S D, STJOHN D H. An analytical model for constitutional supercooling-driven grain formation and grain size prediction [J]. Acta Materialia, 2010, 58(9): 3262-3270. DOI: 10.1016/j.actamat.2010.01.052.
[31] YU Bao-biao, YAN Hong, ZHU Jian-bin., LIU Jian-long, LI Huo-gen, NIE Qiao. Effects of La on microstructure and corrosion behavior of AlSi5Cu1Mg alloy [J]. Acta Metallurgica Sinica (English Letters), 2018, 32(4): 443-451. DOI: 10.1007/s40195-018-0782-9.
[32] SACCONE A, CACCIAMANI G, MACCIO D, BORZONE G, FERRO R. Contribution to the study of the alloys and intermetallic compounds of aluminium with the rare-earth metals [J]. Intermetallics, 1998, 6(3): 201-215. DOI: 10.1016/s0966-9795(97)00066-6.
(Edited by FANG Jing-hua)
中文导读
添加稀土Yb及热挤压后铸态ADC12合金的微观组织及腐蚀行为
摘要:通过光学显微镜(OM)、扫描电子显微镜(SEM)、能谱仪(EDS)和X射线衍射(XRD)研究了稀土Yb的添加及热挤压对铸态ADC12合金的微观组织及腐蚀性为的影响。结果表明,添加0.9%Yb后,合金中的Si相和β-Al5FeSi相都得到了显著的细化,并且还获得了Al3Yb金属间化合物。热挤压后的稀土合金的Si相、β-Al5FeSi相以及稀土相进一步细化。浸泡腐蚀实验和电化学实验发现,热挤压后稀土铝合金的腐蚀电流密度(8.56 μA/cm2)比未处理合金的(17.33 μA/cm2)低50.6%,极化电阻(9252 Ω·cm2)比未处理合金的(2654 Ω·cm2)高71.3%。热挤压及稀土Yb的添加在不同程度上细化了腐蚀微电池中的阴极相,从而使得腐蚀过程中的阴极反应速率降低。
关键词:ADC12;稀土Yb;热挤压;微观组织;耐腐蚀性
Foundation item: Project(51965040) supported by the National Natural Science Foundation of China; Project(20181BAB206026) supported by the Natural Science Foundation of Jiangxi Province, China
Received date: 2019-07-19; Accepted date: 2019-12-20
Corresponding author: YAN Hong, PhD, Professor; Tel: +86-13667090600; E-mail: hyan@ncu.edu.cn; ORCID: 0000-0003-1835-3675
Abstract: The effects of rare earth ytterbium (Yb) addition and hot extrusion on the microstructure and corrosion behavior of as-cast ADC12 were studied by optical microscopy (OM), scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS) and X-ray diffraction (XRD). The experimental results demonstrate that both the Si phase and β-Al5FeSi phase in the alloy with 0.9 wt% Yb have been remarkably refined, and the Al3Yb intermetallic compound has also been obtained. The Si, β-Al5FeSi, and rare earth phases are further refined in the alloy at 0.9 wt% Yb and hot extrusion. The results of the immersion corrosion tests and electrochemical experiments show that the corrosion current density (8.56 μA/cm2) of the alloy with 0.9 wt% Yb addition and hot extrusion is 50.6% lower than the untreated alloy (17.33 μA/cm2), and the polarization resistance (9252 Ω·cm2) was 71.3% higher than the untreated alloy (2654 Ω·cm2). The corrosion in the cathode phase in the micro-battery was refined to varying degrees attributable to the addition of Yb and hot extrusion, where the cathode reaction in the corrosion process caused a decrease of the corrosion rate.


