
Effect of stoichiometric ratio upon electrochemical properties of AB5-type alloys
YU Li-min(于丽敏), JIANG Wen-quan(蒋文全), JIANG Li-jun(蒋利军), FU Zhong-zhen(傅钟臻), ZHANG Wen-guang(张文广)
General Research Institute for Nonferrous Metals, Beijing 100088, China
Received 20 April 2006; accepted 10 June 2006
Abstract:
In order to evaluate the effect of stoichiometric ratio upon electrochemical properties of AB5-type hydrogen storage alloys, a series of alloys Mm0.8La0.2(Ni4.0Mn0.5Al0.3Co0.37Fe0.13)a (a=0.90-1.08) were prepared and the electrochemical properties were tested. Mm0.8La0.2Ni4.0Mn0.5Al0.3Co0.37Fe0.13 was one of some new low cobalt AB5-type hydrogen storage alloys which had been researched to have good electrochemical properties. The results show that the stoichiometric ratio has great effects on the electrochemical properties of alloys. And the effects were investigated in detail. When stoichiometric ratio x<5.3, the activation performances of alloys are all good. But when stoichiometric ratio x>5.3, the activation performances are decreased evidently. When stoichiometric ratio x<5.3, the discharge capacities of alloys are linearly increased with the increase of stoichiometric ratio. When stoichiometric ratio x>5.3, the discharge capacities of hydrogen storage alloys decrease linearly rapidly by the raise of stoichiometric ratio. Overstoichiometry is good to the cycle lives of alloys. When stoichiometric ratio is between 4.8 and 5.4, voltage platforms of alloys tend to increase linearly with the increasing of x. When x>5.4, the increase of stoichiometric ratio leads to the decrease of voltage platform of alloys.
Key words:
hydrogen storage alloys; AB5; low cobalt; stoichiometric ratio; electrochemical properties;
1 Introduction
At present, most of AB5-type hydrogen storage alloys for commercial use are non-stoichiometric ratio ones[1-8]. Since stoichiometric ratio has important effect on the electrochemical properties of alloys, focus has been placed on research about stoichiometric ratio. HIGASHIYAMA et al[1] found that the cycle lives of Mm(Ni3.8Al0.2Mn0.6)(x-0.4)/4.6Co0.4 alloy decreased with increase of x. But NOTTON et al[2] found that the cycle lives of alloys would be better with the increase of departure degree of stoichiometric ratio. FUKUMOTO et al[3] found that the discharge capacities and activation performances of Mm(Ni3.6Co0.07Mn0.4Al0.3)x decreased when x>1. Then we know that there are not accordant cognition about the effect of stoichiometric ratio upon electrochemical properties of AB5-type alloys.
In this paper, in order to evaluate the effect of stoichiometric ratio upon electrochemical properties of AB5-type hydrogen storage alloys, a series of non-stoichiometric ratio alloys Mm0.8La0.2(Ni4.0Mn0.5- Al0.3Co0.37Fe0.13)a (a=0.90-1.08) were designed and prepared. Mm0.8La0.2Ni4.0Mn0.5Al0.3Co0.37Fe0.13 was one of the new low cobalt AB5-type hydrogen storage alloys which had been researched to have good electrochemical properties[9].
2 Experimental
2.1 Preparation of alloys
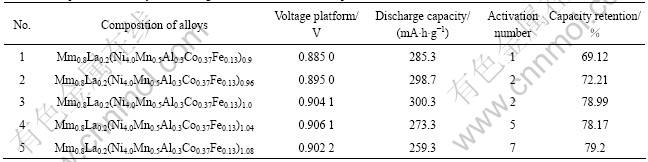
The alloys were melted in an argon atmosphere using a vacuum induction furnace. The alloy ingots were turned over and remelt four times for homogeneity. After induction melting, the melt was poured into a copper mould cooled by water, and a cast ingot was obtained. The chemical compositions of the alloys are Mm0.8La0.2(Ni4.0Mn0.5Al0.3Co0.37Fe0.13)a (a=0.90-1.08). The purity of all the component metals La, Ni, Co, Mn, Al, Fe is at lest 99.5% (mass fraction) and the composition of Mm is La 27.44%, Ce 51.09%, Pr 5.20% and Nd 16.24%.
2.2 Preparation of electrode
The alloys were ground mechanically into powder below 74 μm for the preparation of the experimental electrode. Electrode pellets with 13 mm in diameter were prepared by mixing 0.2 g alloy powder and 0.6 g Ni powder and then compressing under a pressure of 180 MPa. The work electrode was obtained by being enchased in the foam Ni flake and welded with Ni strap.
2.3 Electrochemical measurements
The environment temperature of measurement was kept at 28 ℃. The work electrode was fixed as a negative electrode of a conventional three electrode open-air cell with a sintered Ni(OH)2/NiOOH counter electrode and a reference electrode of Hg/HgO. The electrolyte was 6 mol/L KOH solution, prepared with deionized water. The voltage between the negative electrode and the reference electrode was defined as the discharge voltage. In every cycle, the negative electrode was charged with constant current of 60 mA/g for 6 h, rested for 15min and then discharged at 60 mA/g to the cut-off potential of -0.6 V.
3 Results and discussion
3.1 Electrochemical properties of alloys
The electrochemical properties of the alloys are illustrated in Table 1. Discharge capacities and activation performances of the alloys are shown in Fig.1 and Fig.2, indicating the cycle lives of the alloys.
From Fig.1 and Fig.2 we can find that the electrochemical properties have obvious changes with the difference of the stoichiometric ratio. And it means that the stoichiometric ratio has great effects on the electrochemical properties.
3.2 Effects of stoichiometric ratio on activation performance
Activation performance is one of the important signs of the utility of hydrogen storage alloys. The activation performance is characterized by the initialactivation number. The initial activation number denoted
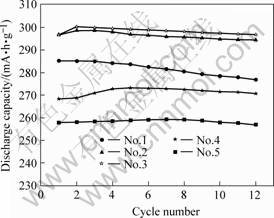
Fig.1 Discharge capacities and activition performances of alloys
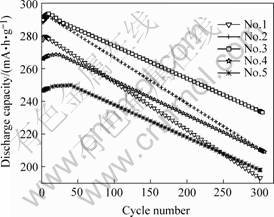
Fig.2 Cycle life curves of alloys
by n is defined as the number of charge-discharge cycles required for attaining the maximum discharge capacity. The charge-discharge cycles were measured with a constant current density of 60 mA/g. With the develop- ment of MH/Ni battery, the initial activation number should be 2 or 3. The relationship between stoichiometric ratio and activation performance is shown in Fig.3. The AB5 dot in Fig.3 is the data of present hydrogen storage alloys for commercial use and other AB5 dot in the figures as follows are all the same.
Table 1 Compositions of alloys and testing results of electrochemical performance
From Fig.3 we can conclude a change tendency of activation performance. When stoichiometric ratio x<5.3, the activation performances of alloys are all good and the activation cycles are less than 3. But when stoichiometric ratio x>5.3, the activation cycles increase greatly to 7 and the activation performance decrease evidently.
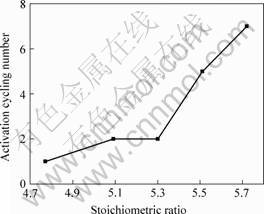
Fig.3 Relationship between stoichiometric ratio and activation performance of AB5
3.2 Effects of stoichiometric ratio on discharge capacity
The maximum discharge capacity is defined as discharge capacity of alloys and measured with a constant current density of 60 mA/g. The discharge capacity is the most important technical parameter of alloys and it is the direct reflection of electrochemical properties. The discharge capacity of hydrogen storage alloys lies on the hydrogen storage quantity of alloys. And the hydrogen storage quantity mainly lies on the crystal lattice spacing. Non-stoichiometric ratio will induce the changes of crystal lattice spacing’s kind, size and amount. And non-stoichiometric ratio can induce the changes of volume of the crystal lattice and microstructure of crystal, too. All these changes can affect the electrochemical properties of alloys.
The relationship between stoichiotric ratio and discharge capacity is illustrated in Fig.4. When stoichiometric ratio x<5.3, the discharge capacities of alloys are linearly increased with the raising of stoichiometric ratio. When stoichiometric ratio x>5.3, the discharge capacity of hydrogen storage alloys decreases linearly with the increase of stoichiometric ratio. Then we know that the stoichiometric ratio has great effect on discharge capacity of alloys. Lack of stoichiometric ratio (x<5.0) means the atom La in side A is partly displaced by Ni and overstoichiometric ratio means the partial replacement of multi-alloys elements in side B. Since the partial displacement of La in side A by Ni leads anisotropy aberration of crystal
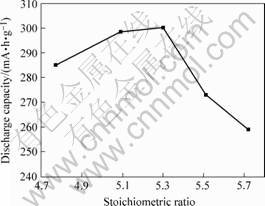
Fig.4 Relationship between stoichiotric ratio and discharge capacity of AB5
lattice, the discharge capacity of alloys is affected greatly. And such does overstoichiometric ratio. Our conclusion about the relationship between stoichiotric ratio and discharge capacity is consistent with the researches of FUKUMOTO[3] and ZHENG[8].
3.3 Effects of stoichiometric ratio on cycle life
The cycle life of alloys can be qualitatively estimated by the charge-discharge cycle characteristics. The decreasing tendency of discharge capacity with the increase of cycle numbers indicates some functional relationship between cycle number and decrease of discharge capacity. The cycle life of alloys is estimated by the capacity retention R after the 300th charge-discharge cycles. The capacity retention R is defined as the ratio of discharge capacity of 300th cycle C300, 300 to the maximum discharge capacity C300, max, i.e. R=C300, 300/C300, max. The cycle life was measured with a constant current density of 300 mA/g. Fig.5 shows the relationship between stoichiometric ratio and cycle life of alloys. It can be derived from Fig.5 that stoichiometric ratio x can affect the cycle life of alloys distinctly. As a whole, the cycle life of alloys took on an increase tendence with the increase of stoichiometric ratio x.
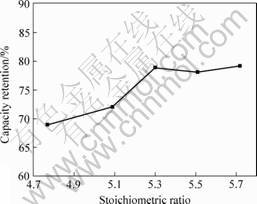
Fig.5 Relationship between stoichiometric ratio and cycle life of alloys of AB5
When x=5.3, the maximum was obtained and then the cycle life tended to stabilization. AB5.3 was the optimizing component which we obtained before[9]. It can be concluded that overstoichiometric ratio is favourable for the cycle stabilities of alloys. This conclusion is consistent with the researches of NOTTEN et al[2] and ZHENG[8].
3.4 Effects of stoichiometric ratio on voltage platform
Discharge voltage platform performance of alloys straightly relates to stoichiometric ratio. Since discharge voltage platform performance directly determines the continuance of 1.2 V discharge voltage plateau of Ni/MH battery, it is also a very important technical parameter which characterizes the utility of alloys. Fig.6 shows the relationship between stoichiometric ratio and voltage platform of alloys.
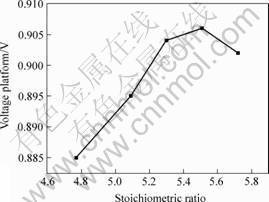
Fig.6 Relationship between stoichiometric ratio and voltage platform of alloys of AB5
When stoichiometric ratio is of 4.8-5.4, voltage platforms of alloys tends to rise linearly with the increase of stoichiometric ratio. So it indicates that the increase of stoichiometric ratio is helpful to improve the discharge kinetics properties of alloys within the range between 4.8 and 5.4. But when x>5.4, the stoichiometric ratio is too excessive to improve the discharge kinetics properties of alloys. When x>5.4, stoichiometric ratio is badly departure from 5 and the cell volumes of alloys would decrease with the rise of stoichiometric ratio x[10-12]. The reducing of cell volume was bad to the storage and diffuseness of hydrogen in alloys and led to the decrease of voltage platform of alloys.
4 Conclusions
1) Stoichiometric ratio has great effects on the electrochemical properties of hydrogen storage alloys. When stoichiometric ratio x<5.3, the activation performances of alloys are all good, the discharge capacities of alloys linearly rise with the increase of stoichiometric ratio. When x>5.3, the discharge capacity of hydrogen storage alloys decrease linearly rapidly as the increase of stoichiometric ratio.
2) With the increase of stoichiometric ratio, the capacity retentions of alloys presented a sharp increasing tendency and tended to stableness at last. Then it could be concluded that overstoichiometry is good to the cycle lives of alloys.
3) When stoichiometric ratio is between 4.8 and 5.4, voltage platforms of alloys tended to rise linearly with the increase of x. When x>5.4, the increase of stoichiometric ratio led to the decrease of voltage platform of alloys.
References
[1] HIGASHIYAMA N, MATSUURA Y, NAKAMURA H, KIMOTO M, NOGAMI M, YONEZU I, NISHIO K. Influence of preparation methods of non-stoichiometric hydrogen-absorbing alloys on the performance of nickel–metal hydride secondary batteries[J]. J Alloys and Compounds, 1997, 253-254: 648-651.
[2] NOTTON P H L, DAAMS J L C, EINERHAND R E F. On the nature of the electrochemical cycling stability of non-stoichiometric LaNi5-based hydride-forming compounds Part Ⅰ. Crystallography and electrochemistry[J]. J Alloys Comp, 1994, 210: 221-241.
[3] FUKUMOTO Y, MIYAMOTO A, MATSUOKA M, IWAKURA C. Effect of the stoichiometric ratio on electrochemical properties of hydrogen storage alloys for nickel-metal hydride batteries[J]. Eletrochimica Acta, 1995, 40(7): 845-848.
[4] HASEGAWA K, OHNISHI M, OSHITANI M, TAKESHIMA K, MATSUMARU Y, TAMURA K. Nickel-Metal hydride battery[J]. Z Phys Chem, 1994, 183: 325-331.
[5] LICHTENBERG F, KOHELER U, FOLAER A, ADKINS N J E, IUTTEL A. Development of AB5 hydrogen storage alloys with low-Co content for rechargeable Ni-MH batteries with respect to electric vehicle applications [J]. J Alloys and Compounds, 1997, 253: 570-579.
[6] L? Jiang-guo, HUANG Jing-yun, YE Zhi-zhen. Optimization of AB5 type hydrogen storage alloys [J]. Metallic Functional Materials, 2001, 8(4): 5-12. (in Chinese)
[7] ZHANG Yang-huan, WANG Xin-lin, CHEN Mei-yan, LI Ping, LIN Yu-fang, LI Rong. Design of chemical compositions on AB5 type hydrogen storage alloy with high quality [J]. Metallic Functional Materials, 2002, 9(5): 1-6. (in Chinese)
[8] ZHENG Qing-jun. Researches about impurities, microstructure and electrochemical properties of hydrogen storage alloys[D]. Beijing: General Research Institute for Nonferrous Metals, 1999: 56-68.
[9] YU L M, JIANG W Q, FU Z Z, JIANG L J, WANG S M. Preparation of low cobalt AB5-type hydrogen storage alloys [J]. Chinese Journal of Rare Metals, 2005, 29(5): 695-699.
[10] ZUTTEL A, CHARTOUNI D, GROSS K, SPATZ P, BACHLER M, LICHTENBERG F, FOLZER A, ADKINS N J E. Relationship between composition, volume expansion and cyclic stability of AB5-type metal hydride electrodes[J]. J Alloys and Compounds, 1997, 253-254: 626-628.
[11] NAKAMURA Y, SATO K, FUJITAI S, NISHIO K, OGURO K, UEHARA I. Lattice expanding behavior and degradation of LaNi5-based alloys [J]. J Alloys and Compounds, 1998, 267: 205-210.
[12] YU Li-min, JIANG Wen-quan, FU Zhong-zhen, JIANG Li-jun. The structure analysis of AB5-type hydrogen storage alloys[J]. Chinese Journal of Rare Metals, 2004, 28(6): 1060-1064. (in Chinese)
Foundation item: Project (2002AA323070) supported by the National High-Tech Research and Development Program of China
Corresponding author: JIANG Wen-quan; Tel: +86-10-82241235; Fax: +86-10-62013148; E-mail: jiangwenquan@grinm.com


