文章编号:1004-0609(2008)S1-0331-05
ZFS共沉积促进剂在复合电沉积过程中的作用机理
范云鹰1,张英杰1, 2,杨显万1,董 鹏1,闫 磊1
(1. 昆明理工大学 材料与冶金工程学院,昆明 650093;
2. 曲靖师范学院 化学与生命科学系,曲靖 650011)
摘 要:
将阳离子型ZFS促进剂与SiO2微粒加入Zn-Fe合金镀液中,制备了Zn-Fe-SiO2复合镀层,研究了ZFS共沉积促进剂对镀层中SiO2微粒沉积量的影响,并分析了促进剂的作用机理。结果表明:ZFS共沉积促进剂可以明显提高SiO2微粒在镀层中的沉积量,并且无需对SiO2微粒进行镀前特殊处理。其原因是该促进剂吸附在SiO2微粒表面,使微粒表面呈正电性,在电场引力的作用下,SiO2微粒容易吸附在阴极表面并与还原金属实现复合共沉积。
关键词:
中图分类号:TG 178 文献标识码:A
Action mechanism of cation-additive ZFS during composite electrodeposition
FAN Yun-ying1, ZHANG Ying-jie1, 2, YANG Xian-wan1, DONG Peng1, YAN Lei1
(1. Faculty of Materials and Metallurgical Engineering, Kunming University of Science and Technology, Kunming 650093, China;
2. Faculty of Chemistry and Life Sciences, Qujing Normal University, Qujing 650011, China)
Abstract: Cation-additive ZFS and SiO2 particles were added into Zn-Fe alloy electrolyte, and Zn-Fe-SiO2 composite coatings were obtained. Effect of additive ZFS on SiO2 content in the coating was studied, and the action mechanism of additive ZFS during composite electrodepositing was analyzed. The results show that additive ZFS can markedly improve the SiO2 content in the composite coating with no pre-treatment on SiO2 particles because SiO2 particles attached with additive ZFS show positive charge and the electric field gravitation between SiO2 particles and cathode makes SiO2 particles absorb onto the cathode and deposit with reductive metal easily.
Key words: composite electrodeposition; additive ZFS; mechanism; adsorption
复合电沉积是通过金属电沉积的方法,将一种或数种不溶于镀液的固体颗粒在阴极与单金属或合金实现共沉积的工艺过程。由于复合镀层的性能具有较大的自由度,因此,近30年来得到了快速发展[1-4]。
微粒共沉积促进剂是一种能对微粒进入镀层起促进作用的添加剂。对于某些复合电镀体系,如果不向镀液中添加微粒促进剂,即使对镀液进行强烈搅 拌,也不能使微粒掺入镀层。另外,有些体系微粒在镀层中的含量较低,需要向镀液中加入微粒促进 剂,以提高镀层中微粒的含量。共沉积促进剂之所以能促进悬浮于镀液中的微粒进入复合镀层,一般与镀液中微粒对促进剂的吸附有密切关系[5-8]。
SiO2微粒是复合电沉积常用的陶瓷颗粒,对金属镀层具有很好的改性效果[9-10]。但是,由于其非导电性与较差的润湿性,它很难在阴极与金属共沉积,需要繁琐的镀前处理工序[11-14]。本文作者从SiO2微粒的吸附特性出发,将自主开发的阳离子型ZFS促进剂与未经镀前处理的SiO2微粒加入Zn-Fe合金镀液中,制备了Zn-Fe-SiO2复合镀层,讨论了ZFS促进剂在复合电沉积过程中的作用机理。
1 实验
采用40 mm×50 mm铜片为阴极,相同面积的0#锌板为阳极,在150 mL矩形槽中进行单面电镀。使用化学纯或分析纯试剂,镀液用蒸馏水配制,溶液pH值用PHS-29A型数字酸度计进行测定。由YH-3010型直流稳压稳流电源提供稳定电流。SiO2微粒平均粒径为0.76 μm ,纯度大于99%,按工艺规范量取后直接加入镀液,边倒入边机械搅拌,搅拌20~40 min待微粒达到充分悬浮即可施镀。施镀过程中采用压缩空气对镀液进行间歇搅拌。
镀液配制:FeSO4·7H2O 30 g/L,ZnSO4·7H2O 50 g/L,(NH4)2SO4 60 g/L,C6H8O7·H2O 30 g/L,抗坏血酸2 g/L,SiO2 30 g/L,ZFS促进剂 0~25 mL/L。
工艺条件:pH 2.5~3.0,电流密度2.5 A/dm2,温度20~30 ℃。
镀层中SiO2的含量用重量法进行测定,具体方法为:
1) 将Zn-Fe-SiO2复合镀层施镀在铜片上,然后将镀层洗净,烘干,冷却后称重,记为W1。
2) 将镀层完全溶解于热盐酸中,镀层中的金属锌和铁成为Zn2+和Fe2+,SiO2微粒成为盐酸中的悬浮颗粒,然后将铜片取出,洗净,烘干,冷却后称重,记为W2。
3) 将盐酸中的SiO2微粒过滤,洗净,烘干,冷却后称重,记为G。
4) 计算镀层中SiO2的质量分数,计算公式为:![]()
2 结果与讨论
表1所列为促进剂ZFS对SiO2在复合镀层中共沉积量的影响。由表1可以看出,促进剂ZFS对SiO2微粒与Zn-Fe合金共沉积的影响十分明显。如果镀液中没有加入促进剂ZFS,则镀层中无SiO2微粒;当镀液中促进剂ZFS添加量逐渐提高时,镀层中SiO2含量也逐渐上升,最后趋于平缓。
表1 促进剂ZFS对SiO2在镀层中共沉积量的影响
Table 1 Effect of additive ZFS on SiO2 content in coating

采用日本EPMA-1600型电子探针测定镀液中促进剂ZFS加入量为15 mL/L时所制备Zn-Fe-SiO2复合镀层的元素分布,如图1所示。

图1 Zn-Fe-SiO2复合镀层元素分布
Fig.1 Elementary distribution of Zn-Fe-SiO2 composite coating: (a) Zn; (b) Fe; (c) Si; (d) O
由图1可知,镀层中存在分布均匀的Zn、Fe、Si及O等元素,说明促进剂ZFS对于形成Zn-Fe-SiO2复合镀层有促进作用。
促进剂ZFS属于阳离子型添加剂,容易吸附在SiO2微粒表面,使微粒与镀液界面间形成双电层。从宏观上来看,SiO2微粒表面的双电层不同于一般的电极双电层,它呈球形,如图2(a)所示;从微观上来看,因为本研究所使用SiO2微粒的粒径为0.76 μm,而一般阳离子的半径为(30~170)×10-12 m[15]。所以,对于每一小部分阳离子来说,SiO2微粒的表面可以看作一个平面,这部分双电层的构造应该与金属电极的双电层相似,也由紧密层和扩散层构成,作者定义其为微双电层,如图2(b)所示。
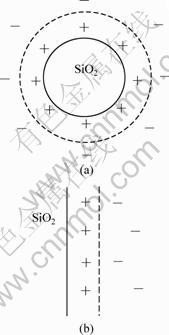
图2 SiO2微粒与镀液界面间的双电层示意图
Fig.2 Double layer formation between surface of SiO2 particle and electrolyte: (a) Macro double layer; (b) Micro double layer
根据电化学理论[16],如果溶液较稀,溶液中各物质的热运动较强,则吸附离子的扩散层较厚,微双电层的构造及静电电位如图3(a)所示;如果溶液较 浓,溶液中各物质的热运动相对减弱,则吸附离子的扩散层变薄,微双电层的构造及静电电位如图3(b)所示;如果溶液很浓并且溶液中的阴离子发生特性吸附,则阴离子的排布很紧密,这时静电电位可以达到负值,微双电层的构造及静电电位如图3(c)所示。
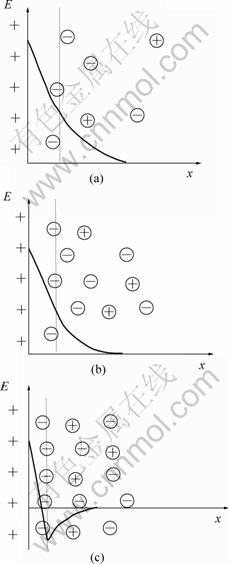
图3 SiO2微粒与镀液界面间的微双电层及静电电位示意图
Fig.3 Structure and distribution of static potential of micro double layer between surface of particle and electrolyte: (a) Dilute electrolyte; (b) Denser electrolyte; (c) Much denser electrolyte
镀液中的阴离子主要是![]() 、OH-离子等,由 于
、OH-离子等,由 于![]() 离子的吸附能力很弱,很难发生特性吸附[16],而OH-离子的浓度小于1×10-11 mol/L(因为镀液的pH值在2~3之间),浓度很低,所以,SiO2微粒表面的微双电层不可能是图3(c)的情况。在图3(a)或(b)的情况下,SiO2微粒表面微双电层中正、负离子间的场强较弱,当SiO2微粒与基质金属在阴极共沉积时,微双电层中容易介入Zn2+、Fe2+等金属阳离子或者其他物质,所以,SiO2微粒容易在阴极被还原金属包覆,从而实现复合共沉积。
离子的吸附能力很弱,很难发生特性吸附[16],而OH-离子的浓度小于1×10-11 mol/L(因为镀液的pH值在2~3之间),浓度很低,所以,SiO2微粒表面的微双电层不可能是图3(c)的情况。在图3(a)或(b)的情况下,SiO2微粒表面微双电层中正、负离子间的场强较弱,当SiO2微粒与基质金属在阴极共沉积时,微双电层中容易介入Zn2+、Fe2+等金属阳离子或者其他物质,所以,SiO2微粒容易在阴极被还原金属包覆,从而实现复合共沉积。
进一步分析可知,当SiO2微粒在阴极表面与基质金属发生共沉积时,会受到镀液的冲击力F及基质金属的结晶压力f作用,这两种力的存在都促使SiO2微粒离开阴极,不利于共沉积。但由于SiO2微粒吸附了阳离子型促进剂ZFS而显正电性,所以,它在阴极表面同时受到阴极的吸引力N,此吸引力使SiO2微粒在阴极表面停留并被不断沉积的基质金属包裹到镀层中,如图4(a)所示。如果SiO2微粒没有吸附一定量的阳离子,那么即使它由于搅拌的作用被传送到阴极表面,它在阴极表面既受到镀液的冲击力,又受到基质金属的结晶压力,如图4(b)所示。这两种力共同作用容易将吸附在阴极表面的SiO2微粒挤走,很难形成复合共沉积。

图4 SiO2微粒在阴极附近的受力
Fig.4 Received force of SiO2 particle near cathode: (a) SiO2 particle attached with additive ZFS; (b) SiO2 particle without additive ZFS
3 结论
1) ZFS共沉积促进剂可以明显提高SiO2微粒在镀层中的沉积量,并且无需对SiO2微粒进行镀前特殊处理。
2) 促进剂ZFS可以吸附在SiO2微粒表面使微粒显正电性,在电场引力的作用下,SiO2微粒容易吸附在阴极表面并与还原金属实现复合共沉积。
REFERENCES
[1] WANG Yun-yan, PENG Wen-jie, CHAI Li-yuan, SHU Yu-de. Electrochemical behaviors of Zn-Fe alloy and Zn-Fe-TiO2 composite electrodeposition[J]. Journal of Central South University of Technology, 2003, 10(3): 183-189.
[2] 常立民, 安茂忠, 石淑云. 复合镀研究的新进展[J]. 吉林师范大学学报: 自然科学版, 2005, 26(2): 13-16.
CHANG Li-min, AN Mao-zhong, SHI Shu-yun. Progress in studies of composite coatings[J]. Jilin Normal University Journal: Natural Science Edition, 2005, 26(2): 13-16.
[3] 王周成, 倪永金, 唐 毅. 电化学方法制备金属基复合材料研究进展[J]. 材料导报, 2006, 20(7): 51-53, 57.
WANG Zhou-cheng, NI Yong-jin, TANG Yi. Research progress in the fabrication of metal matrix composites by electrochemical methods[J]. Materials Review, 2006, 20(7): 51-53, 57.
[4] 周海飞, 杜 楠, 赵 晴. 复合电沉积工艺研究现状[J]. 电镀与涂饰, 2005, 24(6): 41-46.
ZHOU Hai-fei, DU Nan, ZHAO Qing. Status of composite electrodeposition techniques[J]. Electroplating & Finishing, 2005, 24(6): 41-46.
[5] CELIS J P, ROOS J R. Kinetics of the deposition of alumina particles from copper sulphate plating baths[J]. J Electrochem Soc, 1977, 124: 1508-1511.
[6] BERKH O, BODNEVAS A, ZAHAVI J. Effect of additives on electrodeposition of composite chromium coatings[J]. Plating and Surface Finishing, 1994(3): 62-64.
[7] WHITE C, FOSTER J. A study of particle-cathode adhesion during the formation of electrodeposited composite coatings[J]. Transactions of the Institute of Metal Finishing, 1978, 56: 92-96.
[8] 范云鹰, 张英杰, 董 鹏, 罗少林. 复合电沉积的影响因素[J]. 电镀与涂饰, 2007, 26(10): 4-7.
FAN Yun-ying, ZHANG Ying-jie, DONG Peng, LUO Shao-lin. Influencing factors of composite plating[J]. Electroplating & Finishing, 2007, 26(10): 4-7.
[9] TOLUMOYE J, TUAWERI, WILCOX G D. Behaviour of Zn-SiO2 electrodeposition in the presence of N, N-dimethyldodecylamine[J]. Surface and Coatings Technology, 2006, 200: 5921-5930.
[10] FAN Yun-ying, ZHANG Ying-jie, DONG Peng. Preparation and application of electrodeposited Zn-Fe-SiO2 composite coatings[J]. Key Engineering Materials, 2008, 373/374: 212-215.
[11] 郭会清, 方 红, 禹建鹰. 复合镀中分散微粒共沉积的若干问题探讨[J]. 中原工学院学院, 2002, 13(1): 29-31.
GUO Hui-qing, FANG Hong, YU Jian-ying. Problems in coexisted deposition of dispersing particles in composite plating[J]. Journal of Zhongyuan Institute of Technology, 2002, 13(1): 29-31.
[12] TOMASZEWSKI T W, TOMASZEWSKI L C, BROWN H. Codeposition of finely dispersed particles with metals[J]. Plating, 1969, 56(10): 1234-1239.
[13] 陈 亚, 李士嘉, 王春林, 蔡建宏. 现代实用电镀技术[M]. 北京: 国防工业出版社, 2003.
CHEN Ya, LI Shi-jia, WANG Chun-lin, CAI Jian-hong. Modern applied plating technology[M]. Beijing: National Defence Industry Press, 2003.
[14] WHITE C, FOSTER J. Factors affecting the entrapment of alumina particles during the electrodeposition of copper[J]. Transactions of the Institute of Metal Finishing, 1981, 59: 8-12.
[15] 王致勇, 董松琦, 张庆芳. 简明无机化学教程[M]. 北京: 高等教育出版社, 1988.
WANG Zhi-yong, DONG Song-qi, ZHANG Qing-fang. Compendious textbook of abiochemistry[M]. Beijing: Higher Education Press, 1988.
[16] 李 荻. 电化学原理[M]. 北京: 北京航空航天大学出版社, 1999.
LI Di. Electrochemical theory[M]. Beijing: Beijing University of Aeronautics and Astronautics Press, 1999.
基金项目:教育部博士点基金资助项目(20050674007);云南省自然科学基金资助项目(2006B0048M)
通讯作者:范云鹰,副教授,博士;电话:13608862351;E-mail: yunyingfan7739@sina.com


