
Experimentation and thermodynamic modelling on SrZrO3
GONG Wei-ping(龚伟平), CHEN Teng-fei(陈腾飞), LIU Yong(刘 咏),
LI Da-jian(李大建), JIN Zhan-peng(金展鹏), HUANG Bai-yun(黄伯云)
State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 17 January 2007; accepted 10 April 2007
Abstract:
Experimental data for the perovskite phase SrZrO3 were subjected to a critical thermodynamic assessment using the CALPHAD approach. Special attention was paid to the structural behavior of SrZrO3 to illustrate, how to select an appropriate thermodynamic model based on crystal structure and chemistry information, how to identify and resolve the inconsistency between various kinds of experimental data, and how to use thermodynamic modeling as a basic tool in the development and optimization of materials and process. Our assessment results in a Gibbs energy function covering the temperature range between 300 K and the melting point, which explains the experimental data within the experimental uncertainty.
Key words:
thermodynamic modelling; CALPHAD; SrZrO3; experimentation; interaction;
1 Introduction
SrZrO3 has been the subject of several investigations because of its technological applications. For instance, SrZrO3 can be used in the electronic industry as insulators, and its refractory properties are of interest in high-temperature applications. In nuclear safety studies, SrZrO3 plays an important role as it is formed in the UO2 fuel by reaction between the fission products in the fuel matrix, and during core-concrete interactions between the fission products and the oxidized zircaloy cladding. The thermodynamic properties, the structural variants and phase transitions are thus of special interest as they influence the behaviour of hazardous fission products.
While due to the slight distortion, impurities, minor departures from nominal stoichiometry, or changes in synthesis temperatures, great discrepancy existed among the reported experimental data. In this way, thermodynamic modeling on SrZrO3 was carried out in the present study, aiming to validate experimental results from literature and to provide a set of consistent thermodynamic parameters.
2 Evaluation of experimental data in literature
2.1 Structural information
There is continuous interest in the structural variants and phase transitions in SrZrO3, associated with the technological application. However, because of the slight distortion, it has been argued that impurities, minor departures from nominal stoichiometry, or changes in synthesis temperatures could result in different crystal symmetries and phase transformations of SrZrO3, thus two different reviews about the crystallographic structure of SrZrO3 existed in Refs.[1-12]. One was that the room temperature structure of SrZrO3 was pseudo-cubic[1-4], and this pseudo-cubic structure did not undergo any phase transformation upon heating[2-3]. The second review was that the room temperature structure of SrZrO3 was orthorhombic[5-12], and the orthorhombic perovskite SrZrO3 (o-SrZrO3), in Pnma, will transform through higher symmetries during heating, eventually to ideal cubic (c-SrZrO3)[9-13]. Very early, MEGAW[1], SMITH and WELCH[2] indexed the room temperature structure of SrZrO3 to pseudo-cubic. MATHEWS et al[3] failed to detect any phase transformation of pseudo-cubic SrZrO3 in the temperature range between 298 and 1 675 K by high-temperature XRD, ROOSMALEN et al[4] owned the transformations of the pseudo-cubic samples to the impurities and lattice defects governed by the preparation temperature. On the other hand, very good start work on the structural transformations of SrZrO3 was carried out by CARLSOON[8], who adopted XRD and differential thermal analysis(DTA) methods. The observations of CARLSOON[8] as to the sequence of phases were summarized as: o-SrZrO3 (orthorhombic)→p-SrZrO3 (pseudo-tetragonal)→t-SrZrO3 (tetragonal)→c-SrZrO3 (cubic) at 1 000, 1 130 and (1 430±25) K. The occurrence of the structure transformations has been confirmed [9-11]. Very recently, KENNEDY and HOWARD[9] detected the sequent structure transformations by a high-resolution neutron powder diffraction method. YAMANAKA et al[10] and LIGNY and RICHET[11] suggested the transformation temperatures based on the specific heat anomalies in the cp curve of SrZrO3. These heat capacities were determined by differentiation of the least square fits of the experimental enthalpies. The very small enthalpies and entropies attached to the phase transformations were practically determined by LIGNY and RICHET[11] and JOCAB and WASEDA [12]. Because the experimental procedures were well controlled and the experimental results were generally consistent with each other[5-12], the second review about the structure of SrZrO3 was accepted in this work.
2.2 Thermodynamic information
Several scholars[10-22] have investigated the thermodynamic properties of SrZrO3. Using high- temperature differential calorimeter, NAGARAJAN et al[13] reported enthalpy increments (HT-H298) for SrZrO3 from 1 030 to 1 687 K. Drop calorimetry was used by several investigators[14-16], for (HT-H298) measurements on SrZrO3 from 300 K to 1 650 K, 526 K to 2 318 K, and 474.4 K to 906.3 K, respectively. CORDFUNKE and KONINGS[17] have also reported enthalpy values for SrZrO3 in their compilations. GOSPODINOV and MARCHEV[18] reported enthalpy data for SrZrO3 in the temperature range between 298 and 500 K by differential scanning calorimeter(DSC), and BANERJEE et al[19] determined (HT-H298) of SrZrO3 by a precise high-temperature Calvet micro- calorimeter in the temperature range from 384.8 to 991 K. The crystallographic structure and purity of the samples used by these were not described very clearly, while in view of the preparation temperature, it seems most likely that their samples were orthorhombic. These experimental data[13-16, 19] were in good agreement with each other and joined smoothly with the adiabatic measurements carried out by KING and WELLER[20]. Thus they were used to evaluate the Gibbs energy of orthorhombic SrZrO3, o-SrZrO3. Data from GOSPODINOV and MARCHEV[18] were too high to have any physical meaning and were excluded in this work. For the first time, LIGNY and RICHET[11] did systematic measurements on the enthalpy increment (HT-H273) and heat capacities of SrZrO3, and their sample underwent the structure transformation from orthorhombic through tetragonal and eventually to ideal cubic in the temperature range from 300 to 1 800 K. The measured data by LIGNY and RICHET[11] were used to evaluate the Gibbs energy functions of o-SrZrO3, p-SrZrO3, t-SrZrO3 and c-SrZrO3.
The estimated thermodynamic properties of SrZrO3 at 298.15 K, i.e. the enthalpy of formation ?fH298= (-1767.5±3) kJ/mol[12], heat capacity cp(298 K)= (103.43±0.31) J/(mol?K)[20] and standard entropy ![]() =(115.1±0.84) J/(mol?K)[20] were adopted so that the evaluation can be carried out practically, but a low weight factor was applied to them.
=(115.1±0.84) J/(mol?K)[20] were adopted so that the evaluation can be carried out practically, but a low weight factor was applied to them.
The Gibbs energy of formation of SrZrO3 relative to the pure oxides in the temperature range from 960 to 1 210 K, and from 1 182 to 1 364 K have been determined by JOCAB and WASEDA[12] and LEVITSKII[21] using electromotive force, respectively. JOCAB and WASEDA[12] also reported the enthalpies of formation for SrZrO3 from the component oxides in the temperature range from 960 to 1 210 K. The enthalpy values reported by JOCAB and WASEDA[12] were in good agreement with those reported by MUROMACHI and NAVROTSKY[22] at 1 060 K. These data were not used in the optimization. However, they were compared with the calculated results in order to check the final modelling.
3 Thermodynamic models
The Gibbs energy function ![]() =
= ![]() -
- ![]() for the component i (i = ZrO2, SrO) in the phase Φ is expressed by Eqn.(1):
for the component i (i = ZrO2, SrO) in the phase Φ is expressed by Eqn.(1):
![]() =a+bT+cT lnT+dT 2+eT –1+f T 3+gT 7+hT–9 (1)
=a+bT+cT lnT+dT 2+eT –1+f T 3+gT 7+hT–9 (1)
where ![]() is the molar enthalpy of the component i at 298.15 K and 101 325 Pa in its standard element reference(SER) state, and T is the absolute temperature. The last two terms in Eqn.(1) are used only outside the ranges of stability[23], the term gT7 is relative to the liquid below the melting point and hT –9 to the solid phases above the melting point.
is the molar enthalpy of the component i at 298.15 K and 101 325 Pa in its standard element reference(SER) state, and T is the absolute temperature. The last two terms in Eqn.(1) are used only outside the ranges of stability[23], the term gT7 is relative to the liquid below the melting point and hT –9 to the solid phases above the melting point.
In the present work, the Gibbs energy functions of pure ZrO2, ![]()
![]()
![]() and
and ![]() were taken from the assessments of DU et al [24]. The Gibbs energy functions of SrO,
were taken from the assessments of DU et al [24]. The Gibbs energy functions of SrO, ![]() and were taken from SGTE accepted substance database[25].
and were taken from SGTE accepted substance database[25].
Since there were experimental thermodynamic data for o-SrZrO3 in a wide temperature range[14-25], it was preferable to express the Gibbs energies relative to the SER state, and the following equation was used:
![]() =a1+b1T+c1T lnT+d1T 2 +e1T –1 (8)
=a1+b1T+c1T lnT+d1T 2 +e1T –1 (8)
where the coefficients c1, d1 and e1 can be evaluated based on the selected experimental enthalpy increments [14-19, 22] and heat capacities[11, 13, 15].
The Gibbs energies of p-SrZrO3, t-SrZrO3 and c-SrZrO3 were evaluated based on those of o-SrZrO3, neglecting any difference of heat capacity between these forms. The following equations were found sufficient to describe the solid-solid phase transformations of SrZrO3 as well as the corresponding experimental data:
![]() =
=![]() +ΔH1-T·ΔS1 (9)
+ΔH1-T·ΔS1 (9)
![]() =
=![]() +ΔH2-T·ΔS2 (10)
+ΔH2-T·ΔS2 (10)
![]() =
=![]() +ΔH3-T·ΔS3 (11)
+ΔH3-T·ΔS3 (11)
where ΔHi and ΔSi (i=1, 2, 3) are the enthalpies and entropies of the transformations from o-SrZrO3 to p-SrZrO3, from p-SrZrO3 to t-SrZrO3, and from t-SrZrO3 to c-SrZrO3, respectively, which were evaluated by using the corresponding thermodynamic data[13-15].
4 Optimization procedure
The optimization was conducted using the Thermo-calc software package[26]. The critically selected experimental data were processed with a specific weight factor reflecting the experimental uncertainty. The optimization process consists of four steps.
In the first step the measured heat capacities and enthalpy increments were fitted[11, 13-16, 19]. In the next step the Gibbs energy functions of o-SrZrO3 from room temperature up to about 1 023 K were constructed by using the enthalpy of formation and the entropy values at 298.15 K[12, 20]. In the third step the Gibbs energy function of o-SrZrO3 for the derivation of the Gibbs energy of p-SrZrO3, t-SrZrO3, c-SrZrO3 and the enthalpy and temperature of transformation in the analysis of thermodynamic properties[13-22] and structural behavior were obtained[4-12]. To achieve this,
the reported enthalpies and entropies of the transformations from o-SrZrO3 to p-SrZrO3 and from p-SrZrO3 to t-SrZrO3 were included[11-12]. By considering the solid-state transformation between the tetragonal and cubic structure, a small value of 1 J/(mol?K) was assumed for the corresponding entropy change. The Gibbs energy expressions obtained in this way are suitable for explaining the selected thermodynamic properties and structural information and making predictions of thermodynamic properties in multi-component systems. In the last step all model parameters were assessed simultaneously in a least squares optimization to represent the key experimental data within experimental uncertainty.
5 Results
The model parameters obtained from the optimization process are presented in Table 1. These parameters together with evaluated Gibbs energy functions for ZrO2[24], and SGTE recommended Gibbs energies for SrO[25] allow the calculation of thermodynamic and structural properties of SrZrO3. In Table 1 the calculated enthalpy and entropy of SrZrO3 at 298.15 K are also presented. To enhance a comparison with experimental data, the enthalpy and entropy of transformations are calculated, the results of which are presented in Table 1, too. Figs.1 and 2 show calculated (HT-H298) and cp of SrZrO3, respectively. In order to check the final modeling parameters, the Gibbs energy and enthalpy of formation of SrZrO3 relative to the pure oxides in some key temperatures are calculated and listed in Tables 2 and 3, respectively.
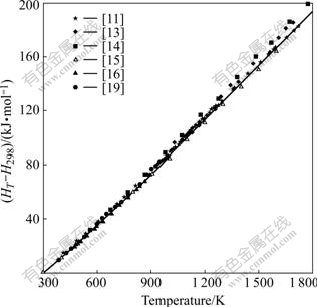
Fig.1 Calculated (HT-H298) of SrZrO3 compared with measured data[11,13-16,19]
Table 1 Summary of thermodynamic properties of SrZrO3 compound

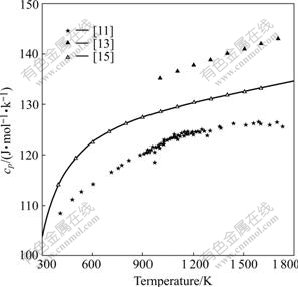
Fig.2 Calculated heat capacity of SrZrO3 compared with measured data[11, 13, 15].
Table 2 Comparison of Gibbs energies of formation of SrZrO3 from component oxides
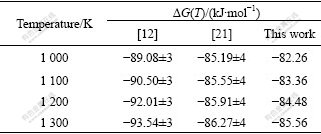
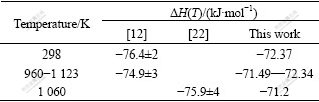
Table 3 Comparison of enthalpy of formation of SrZrO3 from component oxides
6 Discussion
Fig.1 illustrates that our calculated (HT-H298) represents their measurements[11,13-16,19] within experimental uncertainty. Fig.2 indicates that our calculation on cp of SrZrO3 matches quite well with those of NAGARAJAN et al[13] and seems a compromise among the data of LIGNY and RICHET[11] and LEVITSKII et al[15]. Table 1 indicates that our calculated enthalpy and entropy values are consistent with available experimental data. In Tables 2 and 3, the thermodynamic quantities predicted by using the thermodynamic parameters are compared with two pieces of experimental information, which are not used in the modelling. Table 2 illustrates that the predicted Gibbs energy of formation of SrZrO3 relative to the pure oxides reproduce the experimental values[12, 21] within the experimental errors. Table 3 illustrates that the predicted enthalpy of formation of SrZrO3 relative to the pure oxides can reproduce the experimental values of Refs.[12, 22].
In order to check our calculated structural properties of SrZrO3, the compound SrZrO3 was prepared by solid reaction with the suitable ratio of SrCO3 to ZrO2 at 1 723 K. The obtained SrZrO3 were heat-treated at 973 K, 1 123 K and 1 423 K, for 8 h, respectively, followed with air-quenching or furnace-cooling. The samples as- prepared were analysed with XRD to identify the phase structure. As shown in Fig.3, the samples quenched from 1 423 K have cubic structure, and those furnace-cooled have orthorhombic structure, which confirms the polymorphic forms of SrZrO3. While we failed to detect the tetragonal structure from the samples quenched from 1 123 K.

Fig.3 Observed XRD patterns from SrZrO3, showing fundamental perovskite reflections
7 Conclusions
1) The phase transformation sequence of SrZrO3 is estimated: orthorhombic→pseudo-tetragonal→ tetragonal→cubic at 1 007 K, 1 121 K and 1 389 K, respectively. The relative enthalpies and entropies are 1.521 kJ/mol and 1.51 J/(mol?K), 0.686 kJ/mol and 0.612 J/(mol?K), 1.389 kJ/mol and 1 J/(mol?K) in sequence.
2) The thermodynamic function of orthorhombic SrZrO3 is critically evaluated with all reliable experimental data reproduced within the estimated experimental uncertainty. The Gibbs energies of other three structures are estimated based on that of orthorhombic SrZrO3.
References
[1] MEGAW H D. Crystal structure of double oxides of the perovskite type [J]. Proc Phys Soc, 1946, 58: 133-141.
[2] SMITH A J, WELCH A J E. Cation-size control of structure phase transitions in tin perovskites [J]. Acta Crystallogr, 1960, 13: 653- 656.
[3] MATHEWS M D, MIRZA E B, MOMIN A C. High-temperature X-ray diffractometric studies of LaCrO3 [J]. J Mater Sci Lett, 1991, 10: 3246-3248.
[4] VAN ROOSMALEN J A M, VAN VLAANDEREN, CORDFUNKE E H P. On the structure of SrZrO3 [J]. J Solid State Chem, 1992, 101: 59-65.
[5] TILLOCA G, PEREZ M, JORBA Y. X-ray characterization of Sr3Zr2O7 hydrate [J]. Res Intern Haut Temp Refract, 1964, 1: 331- 342.
[6] AHTEE A, AHTEE M, GLAZER A M, HEWAT A W. The structural phase transitions in strontium zirconate revisited [J]. Acta Crystallogr B, 1976, 32: 3243-3246.
[7] KAMISHIMA O, HATTORI T, OHTA K, CHIBA Y, ISHIGAME M. Dielectric relaxation in Yb-doped SrZrO3 [J]. J Phys: Condens Matter, 1999,11: 5355-5365.
[8] CARLSSON L. High-temperature phase transitions in SrZrO3 [J]. Acta Crystallogr, 1967, 23: 901-905.
[9] KENNEDY B J, HOWARD C J. High-temperature phase transition in SrZrO3 [J]. Phy Rev, 1999, 59(6): 4023-4027.
[10] YAMANAKA S, KUROSAKI K, OYAMA T, MUTA H, UNO M, MATSUDA T, KOBAYASHI S I. Thermodynamic properties of perovskite-type strontium cerate and zirconate [J]. J Am Ceram Soc, 2005, 88(6): 1496-1499.
[11] DE LIGNY D, RICHET P. High-temperature heat capacity and thermal expansion of SrTiO3 and SrZrO3 perovskite [J]. Phys Rev B, 1996, 53(6): 3013-3022.
[12] JACOB K T, WASEDA Y. Potentiometric determination of the Gibbs energies of formation of SrZrO3 and BaZrO3 [J]. Metall Mater Trans, 1995, 26B: 775-781.
[13] NAGARAJAN K, SAHA R, BABU R, MATHEWS C K. Thermodynamic functions of barium and strontium zirconates from calorimetric measurements [J]. Therm Acta, 1985, 90: 297-304.
[14] FOMICHEV E N, SLYNSAR N P, KRIVOROTENKO A D, TOLSTAYA V Y. Study of enthalpy of SrHfO3 and SrZrO3 at high temperature [J]. Ogneupory, 1973, 7: 36-37.
[15] LEVITSKII V A, TSAGAREISHVILI D S H, GVELESIANI G G. Enthalpy and specific heat of strontium and barium zirconates at high temperature [J]. High Temp, 1976, 14: 69-72.
[16] HUNTELAAR M E, CORDFUNKE E H P, VAN DER LAAN R R. Heat capacities and enthalpy increments of the metazirconates of calcium, strontium and barium [J]. Therm Acta, 1996, 274: 101-111.
[17] CORDFUNKE E H P, KONINGS R J M. Enthalpy increments of barium zirconate, BaZrO3, and an assessment of its thermochemical properties [J]. Therm Acta, 1989, 156: 45-51.
[18] GOSPODINOV G G, MARCHEV V M. The temperature relations of the thermodynamic quantities of Ca, Sr, Ba, and Pb zirconates [J]. Therm Acta, 1993, 222: 137-141.
[19] BANERJEE A, DASH S, PRASAD R, SOOD D D. Enthalpy increments of strontium and barium zirconates [J]. Therm Acta, 1997, 298: 59-64.
[20] KING E G, WELLER W W. Thermochemistry and thermodynamic properties of substances (1) [M]. Washtington DC, US: US Bur Mines, 1960, 3.
[21] LEVITSKII V A. Thermodynamics of double oxides(I): Some aspects of the use of CaF2-type electrolyte for thermodynamic study of compounds based on oxides of alkaline earth metals [J]. J Solid State Chem, 1978, 25: 9-22.
[22] MUROMACHI E T, NAVROTSKY A. Energetics of compounds (A2+B4+O3) with the perovskite structure [J]. J Solid State Chem, 1988, 72: 244-256.
[23] ANDERSSON J O, GUILLERMET A F, GUSTAFSON P, HILLERT M, JANSSON B, SUNDMAN B, AGREN J. A new method of describing lattice stabilities [J]. Calphad, 1987, 11(1): 93-98.
[24] DU Y, JIN Z P, HUANG P Y. Thermodynamic calculation of the zirconia-calcia system [J]. J Am Ceram Soc, 1992, 75: 3040-3048.
[25] RISOLD D, HALLSTEDT B, GAUCKLER L J. The strontium- oxygen system [J]. Calphad, 1996, 20(3): 353-361.
[26] ANDERSON J O, HELANDER T, HOGLUND L, SHI P, SUNDMAN B. Thermo-Calc l & DICTRA, computational tools for materials science [J]. Calphad, 2002, 26(2): 273-312.
Foundation item: Project(2006AA03Z567) supported by the National High-Tech Research and Development Program of China; Project(33354) supported by the Natural Science Foundation of Guangdong Province, China
Corresponding author: CHEN Teng-fei; Tel: +86-731-8877242; E-mail: tengfei@mail.csu.edu.cn


