
A novel dual nickel coating on AZ91D magnesium alloy
SONG Ying-wei(宋影伟), SHAN Da-yong (单大勇), CHEN Rong-shi (陈容石), HAN En-hou(韩恩厚)
State Key Laboratory for Corrosion and Protection, Institute of Metal Research, Chinese Academy of Sciences,
Shenyang 110016, China
Received 12 June 2008; accepted 5 September 2008
Abstract:
Magnesium alloys covered with metal coating display excellent corrosion resistance, wear resistance, conductivity and electromagnetic shielding properties. The electroless plating Ni-P as bottom layer following the electroplating nickel as surface layer on AZ91D magnesium alloy was investigated. The coating surface morphology was observed with SEM and the structure was analyzed with XRD. Electrochemical tests and salt spray tests were carried out to study the corrosion resistance. The experimental results indicate that the dual coating is uniform, compact and pore-free. The adhesion strength between magnesium alloy substrate and electroless plating Ni-P bottom layer and electroplating nickel surface layer is perfect. The corrosion resistance of AZ91D magnesium alloy is greatly improved after being protected with the dual coating.
Key words:
AZ91D magnesium alloy; dual coating; electroplating Ni; electroless plating Ni-P; corrosion resistance;
1 Introduction
Magnesium and its alloys have a lot of advantageous properties[1-2]. However, the poor corrosion resistance is one of main obstacles for the further wide use of magnesium alloys. Surface treatment technologies can be applied to protecting magnesium alloys from corrosion. The effective protection measurements include chemical conversion, anodizing, electroless plating, electroplating and organic coating and so on[3-7]. Among these surface treatment technologies, metal nickel coating obtained with electroplating or electroless plating is especially popular in the electronic industry because of its good conductivity, electromagnetic shielding, corrosion resistance and wear resistance properties. However, magnesium alloys are “difficult to plate metals” [8-9]. The surface of substrate material will suffer severe attack in the Watts electroplating solution containing Cl- or SO42-. Currently, the metal Ni coating is mainly obtained by electroless plating technology[10-12]. However, in severe corrosive environments, the single electroless plating Ni-P coating cannot provide enough protection to the magnesium alloy substrate[13]. Thus, a novel dual coating is introduced in this work. The electroless Ni-P coating acts as the bottom layer and then the electroplating nickel coating is deposited onto the Ni-P coating. It is expected that the corrosion resistance of magnesium alloys can be greatly improved with the protection of dual coating.
The aim of this work was to prepare a novel dual-layer coating consisting of electroless plating Ni-P layer and electroplating Ni layer, and then the structure and corrosion resistance of the dual coating on the magnesium alloys was investigated.
2 Experimental
The experimental materials used for the investigation were AZ91D magnesium alloy with dimensions of 40 mm×40 mm×2 mm. The chemical compositions are given in Table 1.
Table 1 Chemical compositions of AZ91D magnesium alloy (mass fraction, %)
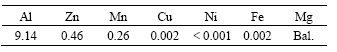
The surface of substrate material was ground with 1000 grit SiC paper to ensure the same surface roughness, and then ultrasonically cleaned in acetone.
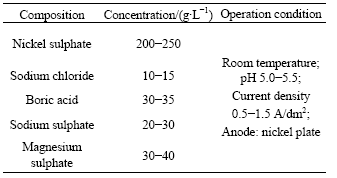
The magnesium alloy substrate was pretreated with alkaline cleaning and activating to remove the greases and oxides prior to the electroless plating. Water rinse was used after every step. The electroless plating solution compositions and operation conditions were as follows: basic nickel carbonate 8-12 g/L, sodium hypophosphite 20-25 g/L, sodium citrate 10-15 g/L, potassium fluoride 5-10 g/L, buffer agent 10-15 g/L, pH 5.5-6.5, and temperature 80 ℃. The solution composition and operation condition of Watts electroplating nickel are shown in Table 2. The toxic chemicals of hydrofluoric acid and Cr6+ were not used in all of the experiments. The whole experimental processes were environmentally friendly.
Table 2 Solution concentration and operation conditions of Watts electroplating nickel
In general, there are not through pores in the Ni-P coating if the coating thickness reaches 12 μm. In this experiment, electroless plating for 2 h following with electroplating for 1 h can ensure the coating without through pores.
The coatings morphologies were observed using Phillips XL30 scanning electron microscope (SEM). The chemical compositions were probed with energy dispersive X-ray spectroscope (EDX).
The crystal structure of electroplating Ni coating was analyzed by X-ray diffractometer (XRD) using a Cu target.
Electrochemical tests were carried out using a classical three-electrode cell with platinum as counter electrode, saturated calomel electrode SCE (+0.242 V vs SHE) as reference electrode, and the samples with an exposed area of 1 cm2 as working electrode. The potentiodynamic curves were measured using a EGG potentiostat model 273 at a constant voltage scan rate of 0.5 mV/s. The scan time for φ—t curve was 1 800 s and the sampling frequency was 5 point/s. The corrosive medium was 3.5% NaCl solution.
Salt spray test was conducted according to ASTMB117 standard (5% NaCl, 35 ℃). The time interval from beginning the testing to the presence of the first corrosion pit (observed with eyes) was used as the appraisement standard. In each case, three samples were tested, and the average value was the final result.
3 Results and discussion
3.1 Surface morphology and chemical composition of coating
The surface morphology of electroless plating Ni-P bottom layer is shown in Fig.1. The coating surface is covered with regular round nodules. There are clear boundaries among nodules. The coating is compact and smooth. Pores are not observed on the surface of Ni-P coating, which ensures that the Ni-P coating cannot be corroded in the Watts electroplating solution containing SO42-. Thus, electroplating Ni can be successfully carried out on the surface of electroless plating Ni-P coating.

Fig.1 Surface morphology of electroless plating Ni-P bottom layer
After the electroplating nickel coating was deposited on the electroless plating Ni-P coating, the surface morphology was observed by SEM as shown in Fig.2. In the case of low magnification morphology of Fig.2(a), the coating consists of round nodules with “cauliflower-like” structure. Compared with the electroless plating Ni-P coating, the dimension of the nodules is large and the surface is coarse. According to the magnified morphology of the nodules in Fig.2(b), the nodules consist of different sizes of subuliform Ni crystal grains.
Fig.3(a) shows the cross-section morphology of the dual coating. It can be found that the coating is compact, uniform and well adhered to the magnesium alloy substrate.
The qualitative analysis of the Ni, P and Mg elements on the cross-section along the line from the substrate to coating surface by EDX is given in Fig.3(b). The composition of phosphorus element changes from zero to a stable concentration then to zero, which represents the magnesium alloy substrate, Ni-P bottom layer and electroplating Ni surface layer, respectively. The thickness of electroless plating Ni-P layer is nearly twice more than that of electroplating Ni layer. The
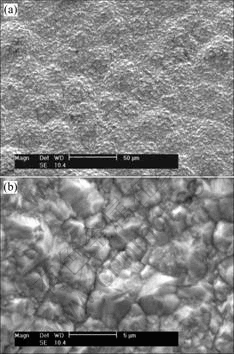
Fig.2 Surface morphology of electroplating nickel surface layer: (a) Low magnification morphology; (b) High magnification morphology
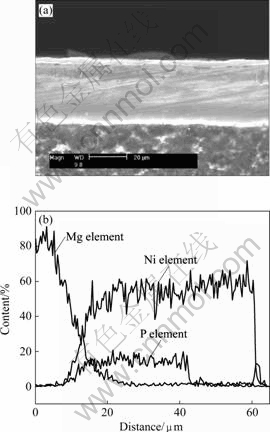
Fig.3 Cross-section morphology and linear distribution of various elements: (a) Cross-section morphology; (2) Line distribution of various elements
possible boundary between the Ni-P bottom layer and electroplating Ni surface layer can be speculated according to the linear distribution of phosphor element. However, there is not boundary observed from the cross-section morphology, indicating the excellent adhesion of the coating. Thus, it is expected that the coating can provide enough protection to the AZ91D magnesium alloy substrate.
3.2 XRD analysis of coating
Fig.4 shows the XRD pattern of the electroplating Ni surface layer. Two sharp diffraction peaks corresponding to (111) and (200) are found, which indicates that the electroplating Ni coating is of complete crystal structure.
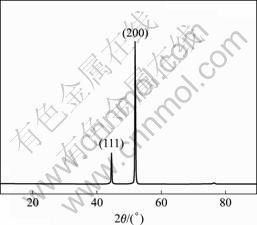
Fig.4 XRD pattern of electroplating nickel surface layer
3.3 Corrosion resistance of coating
The corrosion resistance of AZ91D magnesium alloy with and without the protection of dual coating was compared by φ—t curves and potentiodynamic curves in 3.5% NaCl solution as shown in Fig.5. In the case of φ—t curves, the open circuit potential (φOCP) of AZ91D substrate in 3.5% NaCl solution is only -1.6 V vs SCE. After AZ91D was covered with the dual coating, the φOCP moves toward the more positive potential by about 1.3 V. The magnesium alloy substrate was very active in the NaCl solution. The chemical stability of the substrate material is greatly improved after being deposited with the dual coating. However, the large potential difference between the substrate and coating is also a potential danger. The nickel is a kind of cathodic coating on the anodic magnesium alloy substrate. If there are through pores in the coating, severe galvanic corrosion will destroy the magnesium alloy totally. Thus, it must ensure that there are not any through pore in the coating.
The corrosion behaviors of substrate and coating were studied further by potentiodyanmic curves. The cathodic side of magnesium alloy was controlled with hydrogen evolution reaction. At the beginning of anodic
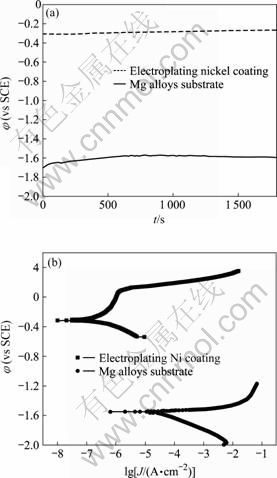
Fig.5 φ—t and potentiodynamic curves of AZ91D magnesium alloy with and without protection of dual coating in 3.5%NaCl solution: (a) φ—t curves; (b) Potentiodynamic curves
side, the corrosion current density increases quickly with increasing anodic potential. Even the Tafel region cannot be found. This indicates that the magnesium alloy substrate suffers serious attack in the NaCl solution. After magnesium alloy is covered with dual coating, there is significant change to the curve. The curve moves toward the left top corner. The cathodic side is oxygen reduction reaction[14]. The anodic side displays the feature of passivation. It keep very low corrosion current density at the potential region from -0.3 V to 0.1 V (vs SCE). When the potential reaches 0.1 V (vs SCE), the corrosion current density increases quickly, indicating the breakdown of the surface coating. Additionally, the free corrosion current density (Jcorr) is approximate 10-6 and 10-4 A/cm2 for the coating and substrate, respectively. This implies that the corrosion rate of magnesium alloy can be reduced by about 100 times after being deposited with dual coating. The corrosion morphology of dual coating after potentiodyanmic curve test is shown in Fig.6. There exist many round pores in the surface of the coating according to Fig.6(a). The
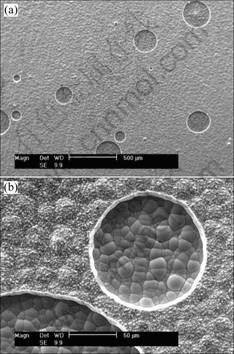
Fig.6 Surface morphologies of dual coating after potentiodynamic curves test: (a) Low magnification morphology; (b) High magnification morphology
diameter of the pores is different, but the pores are in a very regular round shape. The high magnification morphology of the coating in Fig.6(b) can clearly reveal the corrosion morphology near to the corrosion pores. The pores had already penetrated the electroplating Ni surface layer, and smooth nodule surface was exposed. The exposed surface is in accordance with the morphology of electroless plating Ni-P bottom layer. The chemical composition of the exposed Ni-P bottom layer was analyzed with EDX and the result is shown in Fig.7. Only phosphor and nickel elements were included in the
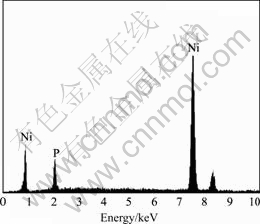
Fig.7 EDX pattern of exposed electroless plating Ni-P bottom layer
pattern. There are not corrosion products on the Ni-P layer. This result indicates that only the electroplating Ni surface layer is broken down at high potential regions during potentiodyanmic curve test and the electroless plating Ni-P bottom layer is undamaged, which can continue to prevent magnesium alloy substrate from corroding. Thus, the dual coating can provide dual protection to the magnesium alloy substrate. The magnesium alloy can be used in the severe corrosive environments safely.
The corrosion resistance of magnesium alloy with the protection of dual coating was tested by salt spray experiment. The coating can protect magnesium alloy from corroding for about 500 h, which is superior to that of the single Ni-P coating of 100 h[15]. To sum up, the dual coating can greatly improve the corrosion resistance of magnesium alloys.
4 Conclusions
1) A novel dual coating of electroplating nickel as surface layer and the electroless plating Ni-P as bottom layer is developed to protect Mg alloy from corroding. The dual coating is uniform, compact and well adhesive.
2) The XRD result shows the complete crystal structure of the electroplating nickel coating.
3) The electrochemical tests and salt spray tests indicate that the corrosion resistance of magnesium alloy is greatly improved with the protection of dual coating, and can be used in the severe corrosive environments safely.
References
[1] GRAY J E, LUAN B. Protective coatings on magnesium and it alloys—A critical review [J]. Journal of Alloys and Compounds, 2002, 336: 88-113.
[2] MAKAR G L, KRUGER J. Corrosion of magnesium [J]. International Materials Reviews, 1993, 38(3): 138-153.
[3] ZHAO M, WU S S, AN P, LUO J R, FUKUDA Y, NAKAE H. Microstructure and corrosion resistance of a chromium-free multi-elements complex coating on AZ91D magnesium alloy [J]. Materials Chemistry and Physics, 2006, 99: 54-60.
[4] ZHANG R F, SHAN D Y, CHEN R S, HAN E H. Effects of electric parameters on properties of anodic coatings formed on magnesium alloys [J]. Materials Chemistry and Physics, 2008, 107: 356-363.
[5] KIM J, WONG K C, WONG P C, KULINICH S A, METSON J B, MITCHELL K A R. Characterization of AZ91 magnesium alloy and organosilane adsorption on its surface [J]. Applied Surface Science, 2007, 253: 4197-4207
[6] SONG Y W, SHAN D Y, CHEN R S, HAN E H. Study on electroless Ni-P-ZrO2 composite coatings on AZ91D magnesium alloys [J]. Surface Engineering, 2007, 23(5): 334-338.
[7] YAMAUCHI N, UEDA N, OKAMOTO A, SONE T, TSUJIKAWA M, OKI S. DLC coating on Mg-Li alloy [J]. Surface and Coatings Technology, 2007, 201: 4913-4918.
[8] AMBAT R, ZHOU W. Electroless nickel-plating on AZ91D magnesium alloy: Effect of substrate microstructure and plating parameters [J]. Surface and Coatings Technology, 2002, 179(1): 124-134.
[9] XIANG Y H, HU W B, LIU X K. Initial deposition mechanism of electroless nickel plating on magnesium alloys [J]. Trans IMF, 2001, 79(1): 30-32
[10] CHEN Y L, YU G, HU B N, LIU Z, YE L Y, WANG Z F. A zinc transition layer in electroless nickel plating [J]. Surface and Coatings Technology, 2006, 201(3/4): 686-690.
[11] JIANG Y F, LIU L F, ZHAI C Q, ZHU Y P, DING W J. Corrosion behavior of pulse-plated Zn-Ni alloy coatings on AZ91 magnesium alloy in alkaline solutions [J]. Thin Solid Films, 2005, 484: 232-237.
[12] ZHANG W X, HE J G, JIANG Z H, JIANG Q, LIAN J S. Electroless Ni-P layer with a chromium-free pretreatment on AZ91D magnesium alloy [J]. Surface and Coatings Technology, 2007, 201: 4594-4600.
[13] SONG Y W, SHAN D Y, HAN E H. High corrosion resistance multilayer nickel coatings on AZ91D magnesium alloys [J]. Surface Engineering, 2007, 23(5): 329-333.
[14] CHAO Chu-nan. Principle of corrosion electrochemistry [M]. Beijing: Chemical Industry Press, 2004. (in Chinese)
[15] SONG Y W, SHAN D Y, CHEN R S, HAN E H. High corrosion resistance of electroless composite plating coatings on AZ91D magnesium alloys [J]. Electrochimica Acta, 2008, 53: 2135-2143.
(Edited by YANG Hua)
Foundation item: Project(2007CB613705) supported by the National Key Basic Research Program; Project(2006BAE04B05-2) supported by the National Key Technology R&D Program of China
Corresponding author: SONG Ying-wei, PhD; Tel: +86-24-23915897; Fax: +86-24-23894149; E-mail: ywsong@imr.ac.cn


