泡沫镍基Ni-S-Co涂层电极在碱性介质中的电催化析氢性能
周科朝,袁铁锤,李瑞迪,何捍卫,黄 冠
(中南大学 粉末冶金国家重点实验室,湖南 长沙,410083)
摘 要:
摘 要:以泡沫镍为基体采用电沉积法制备非晶态Ni-S-Co合金涂层电极。以扫描电镜(SEM)和X射线衍射(XRD)观察表面形貌和微观结构,电化学测试方法分析涂层的电化学行为。结果表明,所获得的镀层为非晶态结构,表面颗粒细小且具有丰富的表面积;非晶态Ni-S-Co合金电极的析氢催化性能较好,与Ni和Ni-S电极相比具有较低的析氢过电位、较高的交换电流密度和较低的表观活化能;经过KOH碱溶液活化处理后,镀层表面颗粒变细,活性表面积增加,析氢性能有所增强。
关键词:
中图分类号:TQ153.2 文献标识码:A 文章编号:1672-7207(2007)02-0186-04
Electrocatalytic properties of Ni-S-Co coatings electrodeposited on nickel foam substrate for hydrogen evolution in alkaline medium
ZHOU Ke-chao, YUAN Tie-chui, LI Rui-di, HE Han-wei, HUANG Guan
(State Key Laboratory of Powder Metallurgy,Central South University, Changsha 410083, China)
Abstract:Amorphous Ni-S-Co alloy coating was prepared by means of chemical electrodepostion method on foam nickel matrix. The surface morphologies and microstructures of Ni-S-Co coatings were observed by SEM and XRD, and the electrochemical properties were tested using electrochemical methods. The results show that the coating is amorphous structure and the particles in surface are fined with large specific surface area. The Ni-S-Co alloy is catalytically more active in comparison with Ni and Ni-S electrode with its lower potential for hydrogen evolution, higher exchange current density and lower activation energy. The hydrogen evolution reaction (HER) is enhanced, the particles in surface are smaller and the surface area is larger after activated by KOH alkaline solution.
Key words:amorphous alloy; electrodeposition; hydrogen evolution reaction; Ni-S-Co coating
氢能作为无污染的生态清洁能源,一直倍受各国科技工作者的关注[1]。大规模、廉价地生产氢是开发和利用氢能的主要环节之一。电解水制氢是实现大规模生产氢的重要手段。但电解水制氢法存在着能耗大、成本高的缺点,最理想的降低能耗的方法就是降低阴、阳极的析氢和析氧过电位。目前,工业上应用的RuO2-TiO2(DSA)阳极过电位已较低(约30 mV),因此,研究新型的阴极材料有效降低阴极过电位具有很重要的现实意义。早期电解水制氢的阴极材料主要以贵金属Pt,Pd及其合金为主,这类合金超电势很低,电催化效果好,但其价格昂贵,难应用于工业生产中。目前,工业上常用的碱性水电解制氢的电极是Raney-Ni电极,但该电极存在强度较差和性能不稳定的问题。其次是Ni-S合金电极,挪威Norsk Hydro公司率先用电沉积的方法在C/Ni基体上制备出了电化学活性非常高的Ni-S电极[1],在25% KOH溶液、80 ℃和电流密度为100 mA/cm2时电解,析氢过电位在6个月内始终维持在60~110 mV。Ni-S阴极可以由多种方法制得,通常在瓦特浴中加入KSCN[2],NaSCN[3]和硫脲[4](TU)等作为硫源。
近来人们研究了d电子层结构的过渡金属间的协同作用,以提高电极材料的析氢性能[5-7]。考虑到Co与Ni同属ⅧB族元素,均为过渡金属,具有相同的电子层结构,决定了两者所形成的化合物也具有相似性。本文作者试图通过向Ni-S镀液[8-11]中添加CoSO4?7H2O,在泡沫镍基体上以电沉积方法制备Ni-S-Co合金电极,并研究这种电极材料的表面微观形貌和结构,通过极化曲线法研究其在碱性介质中的电催化析氢性能。
1 实 验
1.1 Ni-S-Co合金涂层电极的制备
电极制备:阳极为1.5 cm×1.5 cm的镍片,阴极为1 cm×1 cm的泡沫镍,所用镍片及泡沫镍的镍含量均大于99%。电镀液基本组成为瓦特浴体系:硫酸镍60~160 g/L,硫酸钴5~30 g/L,硫脲10~120 g/L,硼酸10~60 g/L,氯化钠5~40 g/L,添加剂和表面活性剂适量。采用双阳极单阴极,在30~60 ℃、电流密度为5~60 mA/cm2和pH值为3~6的条件下进行电沉积。沉积时间为20~100 min。沉积完毕后,样品用去离子水清洗干净后自然干燥。用30% KOH溶液在50~ 90 ℃活化处理1~10 min。
1.2 电极表面形貌和结构
用扫描电镜(KYKY-2800)对镀层表面形貌进行观察,用面能谱扫描分析镀层表面Ni,S和Co元素含量,用X射线衍射(D/MAX-RB)分析镀层物相组成和结晶状态。
1.3 电化学性能测试
用CHI660b(USA)电化学综合分析仪测量涂层的电化学性能,待测电极为工作电极,参比电极为饱和Hg/Hg2Cl2,铂丝为对电极,采用仪器欧姆补偿功能自动校正测试溶液的电位降,电解液为30% KOH水溶液,不同温度下测定其阴极极化曲线。
2 结果与讨论
2.1 电极表面微观结构
图1所示为非晶态Ni-S-Co合金电极的SEM图像。从图1可以看出,泡沫镍基合金涂层电极的孔隙尺寸为0.3~0.4 mm,并具有多层结构。这种特殊的三维多孔结构使其与其他电极相比具有丰富的比表面积;由图中a位置放大5 000倍可看出,镀层表面丰富,有利于提高氢析出反应(hydrogen evolution reaction, HER)催化性能。成分测试结果表明,该合金电极中Ni,S和Co含量分别为78.47%,19.34%和2.19%,表明制得的涂层电极为Ni-S-Co镀层。图2所示为Ni-S-Co合金电极的X射线衍射谱。由图2可看出,非晶态Ni-S-Co镀层具有明显的基体镍峰,同时峰底出现宽化,显示出非晶态特征。

图1 非晶态Ni-S-Co镀层的SEM图像
Fig.1 SEM images of amorphous Ni-S-Co coating

图2 非晶态Ni-S-Co镀层的X射线衍射谱
Fig.2 XRD pattern of amorphous Ni-S-Co coating
2.2 非晶态Ni-S-Co合金电极与其他电极阴极极化曲线的比较
相同条件下电极的析氢电位越低,其析氢活性就越高,电催化性能就越好。图3所示为不同电极在室温(24 ℃)和30% KOH溶液中的动电位扫描极化曲线。由图3可以看出,在相同的电流密度下Ni-S-Co电极的析氢电位远低于Ni-S和Ni电极的析氢电位。如在电流密度为100 mA/cm2下电解时,该合金电极比Ni-S和Ni电极的析氢电位分别降低81 mV和365 mV。表明Ni-S-Co合金电极析氢的电催化活性高于Ni-S和Ni电极电催化的活性,同时也表明在Ni-S合金电极中掺入Co元素能有效提高电极的电催化活性。
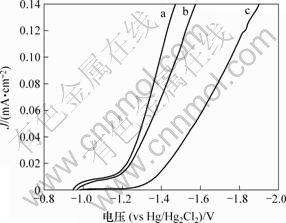
(a) Ni-S-Co; (b) Ni-S; (c) Ni
图3 不同电极在室温(24 ℃)以及30% KOH溶液中的极化曲线
Fig.3 Polarization curves of various electrodes in 30% KOH solution at room temperature(24 ℃)
2.3 电极的表观活化能
3种电极的反应动力学参数如表1所示。由表1可以看出,随着温度的升高,合金电极的交换电流密度J0随之增大,说明工作介质的温度对电极的析氢电位影响较大,提高电解质溶液的温度有利于降低析氢过电位。根据Arrhenius公式,电极析氢反应的真实交换电流密度与表观活化能及温度之间符合如下关系:

表1 3种催化电极的氢气析出反应的动力学参数
Table 1 Kinetics parameters of three different electrodes for HER

根据Tafel曲线求得电极在不同温度下的lgJ0数值,得到lgJ0—1/T关系如图4所示。由图4计算得到Ni电极的表观活化能(![]() )为49.5 kJ/mol,Ni-S电极的表观活化能为40.3 kJ/mol,而Ni-S-Co电极的表观活化能为38.6 kJ/mol。由此可见,Ni-S-Co电极的表观反应活化能低于其他电极的表观反应活化能,说明它的电化学活性高于Ni-S电极和Ni电极的电化学活性。
)为49.5 kJ/mol,Ni-S电极的表观活化能为40.3 kJ/mol,而Ni-S-Co电极的表观活化能为38.6 kJ/mol。由此可见,Ni-S-Co电极的表观反应活化能低于其他电极的表观反应活化能,说明它的电化学活性高于Ni-S电极和Ni电极的电化学活性。
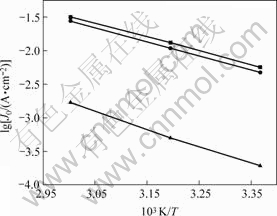
图4 电极材料交换电流密度对数与T -1的关系
Fig.4 Relationship between exchange current density and T -1 of electrode materials
2.4 活化处理对电极HER催化性能的影响
活化处理前后Ni-S-Co涂层合金电极的析氢极化曲线如图5所示。从图5中可看出,经KOH溶液处理后的合金电极的析氢性能有所提高。如在电流密度100 mA/cm2电解时,活化处理后镀层的析氢电位降低了约10 mV。经活化处理后的涂层的SEM图像如图6所示。由图6可以看出,经过KOH碱溶液活化处理后镀层表面分布着细小的球形颗粒,显著提高了电极材料的表面真实面积,从而提高了电极的HER催化性能。
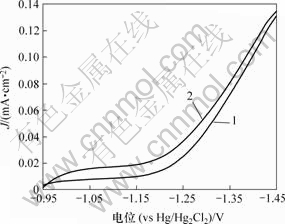
1—活化前; 2—活化后
图5 活化处理前、后非晶态Ni-S-Co电极析氢反应(HER)的影响
Fig.5 HER properties of amorphous Ni-S-Co electrodes before and after activated

图6 活化处理后非晶态Ni-S-Co镀层的SEM图像
Fig.6 SEM image of Ni-S-Co amorphous coating after activated
3 结 论
a. Ni-S-Co镀层为非晶态结构,经过KOH碱溶液活化处理后镀层表面颗粒变细,活性表面积增加,电催化性能增强,在电流密度为100 mA/cm2电解时,活化处理后镀层的析氢电位降低了约10 mV。
b. 非晶态Ni-S-Co合金电极具有较好的析氢催化性能。在电流密度为100 mA/cm2电解时,该合金电极比Ni-S和Ni电极的析氢电位分别降低了81和365 mV。通过实验计算出Ni, Ni-S和Ni-S-Co这3种电极的表观活化能分别为:49.5,40.3和38.6 kJ/mol。
参考文献:
[1] Norsk H O. Electrolyte Cell Active Cathode with low Overvoltage: Nederlands, 7801955[P]. 1978-08-28.
[2] Hine F, Ysuda M, Watanabe M. Studies of the nickel-sulphur electrodeposited cathode[J]. Denki Kagaku, 1979, 47(2): 400-404.
[3] Gonsalez E R, Avaca L A, Tremiliosi-Filho G, et al. Hydrogen evolution reaction on Ni-S electrodes in alkaline solutions[J]. International Journal of Hydrogen Energy, 1994, 19(1): 15-19.
[4] Paseka I. Sorption of hydrogen and kinetics of hydrogen evolution on amorphous Ni-Sx electrodes[J]. Electrochimica Acta, 1993, 38(16): 2449-2454.
[5] 李 宁, 丁大勇, 黎德育. 非晶态Ni-Co-P膜层在碱性介质中的电催化析氢性能[J]. 哈尔滨工业大学学报, 2005, 37(9): 1185-1188.
LI Ning, DING Da-yong, LI De-yu. Electrocatalytic behavior of amorphous Ni-Co-P coating for hydrogen evolution in alkaline medium[J]. Journal of Harbin Institute of Technology, 2005, 37(9): 1185-1188.
[6] Kirk D W, Thorpe S J, Suzuki H. Ni-base amorphous alloys as electro catalysts for alkaline water electrolysis[J]. International Journal of Hydrogen Energy, 1997, 22(5): 493-500.
[7] Trygve B. Hydrogen evolution on NiPX alloys: the influence of sorbed hydrogen[J]. International Journal of Hydrogen Energy, 2001, 26: 1193-1198.
[8] 刘 芳, 何捍卫, 周科朝, 等. 电沉积Ni-S合金硫含量的影响因素[J]. 粉末冶金材料科学与工程, 2005, 10(1): 60-64.
LIU Fang, HE Han-wei, ZHOU Ke-chao, et al. Dependences of sulfur content during electro-deposition of Ni-S alloys[J]. Materials Science and Engineering of Powder Metallurgy, 2005, 10(1): 60-64.
[9] HE Han-wei, LIU Hong-jiang, LIU Fang, et al. Distribution of sulphur and electrochemical properties of nickel sulphur coatings electrodeposited on the nickel foam as hydrogen evolution reaction cathodes[J]. Materials Letters, 2005, 59(10): 3968-3972.
[10] HE Han-wei, LIU Hong-jiang, LIU Fang, et al. Structures and electrochemical properties of amorphous nickel sulphur coatings electrodeposited on the nickel foam substrate as hydrogen evolution reaction cathodes[J]. Surface and Coatings Technology 2006, 201(10): 958-964.
[11] 何捍卫, 刘红江, 刘 芳, 等. 泡沫镍基镍硫合金涂层的形貌、结构和析氢性能研究[J]. 功能材料, 2006, 37(1): 87-91.
HE Han-wei, LIU Hong-jiang, LIU Fang, et al. Study on micro- appearance, structure and properties of hydrogen evolution reaction of nickel-sulphur alloy coatings electrodeposited on the nickel foam substrate[J]. Journal of Functional Materials, 2006, 37(1): 87-91.
收稿日期:2006-10-24
基金项目:国家高技术研究发展计划资助项目(2003AA305980)
作者简介:周科朝(1962-),男,湖南衡阳人,教授,博士生导师,从事功能材料的研究
通讯作者:袁铁锤,男,博士研究生;电话:0731-8877880; E-mail:tiechuiyuan@mail.csu.edu.cn


