Trans. Nonferrous Met. Soc. China 24(2014) 131-135
Effects of Sb content on structure and laser reflection performance of ATO nanomaterials
Jing ZHANG, Li-xi WANG, Min-peng LIANG, Qi-tu ZHANG
College of Materials Science and Engineering, Nanjing Technology University, Nanjing 210009, China
Received 19 December 2012; accepted 6 June 2013
Abstract:
Antimony tin oxide (ATO) nano-particles doped with different Sb contents were prepared by co-precipitation method, using SnCl4·5H2O and SbCl3 as main raw materials. Microstructure, morphology and reflectivity curves were characterized by XRD, FESEM, UV-visible spectroscopy and laser, and the effects of Sb content on crystalline microstructure, crystal size and reflectivity curves of the ATO nano-particles were investigated systematically. The results show that the ATO nano-particles prepared by co-precipitation method have tetragonal rutile structure, with particle size distribution range of several decade nanometer. With the increase of Sb content, the grain size of ATO decreases, and the unit cell volume increases. Compared with the SnO2 particles without Sb, the 1.06 μm laser reflection of ATO nano-particles doped with Sb is obviously lower. With the increase of Sb content, the reflection increases first, then decreases; when the Sb content is 20 %, 1.06 μm laser reflection of ATO nano-particles is below 0.02%, and the laser reflection performance is the best.
Key words:
antimony tin oxide (ATO); Sb content; co-precipitation method; laser reflection;
1 Introduction
With the rapid development of technology and equipments of laser detection and laser guidance, laser stealth technology becomes more and more important in military stealth field. Currently, the laser stealth technology focuses on the wavelength of 1.06, 1.54 and 10.6 μm, scilicet the frequency of near-infrared and mid-infrared, among these 1.06 μm is the major and important working wave length as lasers [1-3]. For laser stealth, using the laser stealth materials is the most effective method [4,5], so exploring and researching on laser absorption on wave length 1.06 μm becomes an important work on laser research [6-8]. Antimony doped tin oxide is the solid solution of Sb-doped SnO2 and one of the n-type metal-semiconductor. The carrier concentration increases by donor atom Sb proportional doping in SnO2, changing the preparation parameters to control the carrier concentration of ATO and changing the absorption range and plasma wavelength can achieve the purpose of laser absorption [9-12]. ATO can be used as good laser absorption because of its strong absorbing and low reflective of 1.06 μm laser.
The optical properties of ATO nano-particles are closely related to the Sb content, therefore, ATO nano- particles with different mole ratios of Sb to Sn will be prepared to research the laser reflection in the 1.06 μm laser. YANG et al [13] used sol-gel method to synthesize ATO nano-particles, and researched the effects of Sb content on the crystalline microstructure, the crystalline size and the resistivity of the ATO nano-particles, but they did not research the reflection of ATO. In this work, we used co-precipitation method to prepare the ATO nanoparticles because it is a simple process and easy to control [14,15], using SnCl4·5H2O and SbCl3 as main raw materials, and NH3·H2O as precipitation agent, and researched the effects of Sb content on the structure and laser reflection performance of ATO nano- particles under certain conditions of the pH, the co- precipitation temperature, the calcination temperature and the calcination time. And the prepared powders were processed by ball milling to provide guidance for practical application of large volume materials.
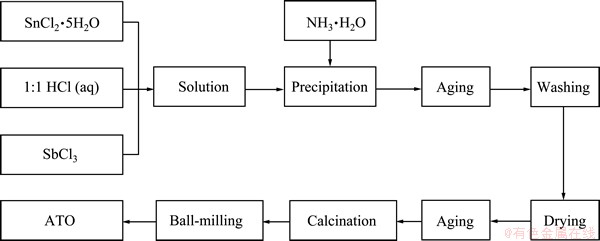
Fig. 1 Process of ATO powders by co-precipitation method
2 Experimental
2.1 Preparation
ATO nano-particles with different mole ratios of Sb to Sn were prepared by co-precipitation, using SnCl4·5H2O and SbCl3 as main raw materials and NH3·H2O as precipitation agent. The preparation flow chart is shown in Fig. 1
2.2 Characterization
The obtained powders were analyzed for their structure, morphology, reflectivity curves and laser property. The X-ray diffraction analysis (XRD, Rigaku D/max 2500) was carried out with an ARL X-ray powder diffractometer using Cu Ka radiation. Surfaces and crystal size of ATO nano-particles were observed employing a field emission scanning electron microscope (FESEM). The reflectance spectra in the wavelength range of 900-1800 nm were measured and the reflection at 1.06 μm laser was recorded by an UV-visible spectroscope.
3 Results and discussion
3.1 Phase analysis
Figure 2 shows the XRD patterns of the ATO powders prepared with different mole ratios of Sb to Sn (0/10, 1/10, 2/10, 4/10 respectively) at the co-precipitation room temperature, pH value 2, and calcination temperature 600 °C. The characteristic diffraction peaks of SnO2 appear in the XRD patterns of ATO nano-particles with different ratios of Sb to Sn, and no other characteristic diffraction peaks appear. The structure of ATO nano-particles is also SnO2 tetragonal rutile structure, but the intensity of SnO2 diffraction peak is lower than that of the ATO without Sb, and the crystal planes (112) and (301) do not appear. The crystallinity of the powders decreases with the doping of Sb. The widening phenomenon of diffraction peaks appears when the mole ratio of Sb to Sn is 2:10. This is because the lattice defect caused by Sb partial replacement of Sn hindered the growth rates of ATO grains.
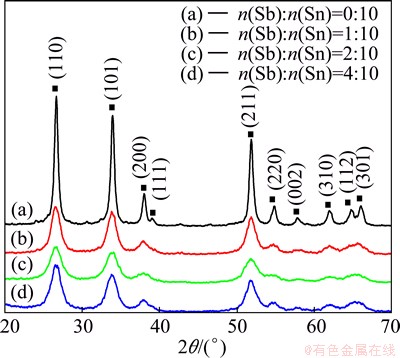
Fig. 2 XRD patterns of ATO powders obtained with various molar ratios of Sb to Sn
The grain sizes of ATO powders obtained with various molar ratios of Sb to Sn gained by the JADE software are shown in Fig.3. The grain size is 24 nm for SnO2 powders without Sb, and decreases to 12 nm when the mole ratio of Sb to Sn is 1/10. The grain size of ATO nano-particles decreases gradually with the increase of mole ratio of Sb to Sn.
Figure 4 shows the FESEM images of ATO nano-particles with the mole ratios of Sb to Sn of 1/10 and 2/10. The structure of ATO nano-particles with different mole ratios of Sb to Sn is approximately spherical. When the mole ratio of Sb to Sn is 1/10, the range of particle size of ATO nano-particles is 40-60 nm. When the mole ratio of Sb to Sn is 2/10, the range of particle size of ATO nano-particles is 30-50 nm.
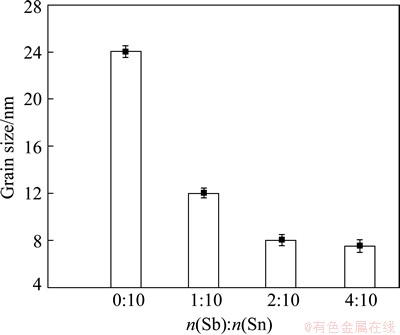
Fig. 3 Grain sizes of ATO powders obtained with various molar ratios of Sb to Sn
3.2 Structural analysis
The effects of Sb content on the structure are studied when the mole ratios of Sb to Sn are 0/10, 1/10, 2/10 and 4/10, respectively. Table 1 shows the comparison of XRD data of ATO with different Sb contents and tetragonal SnO2. Compared with diffraction peaks of tetragonal SnO2, the diffraction peak position of ATO with different Sb contents shifted a small angle due to the fact that the lattice constant of SnO2 changed a little caused by doping Sb. Table 2 shows the variation of lattice constant/unit cell volume of ATO nano-particles with the content of Sb. The unit cell volume of ATO increases gradually with the increase of Sb, due to the increasing of lattice expansion with the increase of Sb.

Fig. 4 FESEM images of ATO particles obtained with various mole ratios of Sb to Sn
3.3 Analysis of laser reflectivity
The laser reflection characteristics of ATO nano-particles with different Sb contents were tested by UV-visible spectroscopy. Figure 5 shows the reflectivity curves of ATO powders obtained with various mole ratios of Sb to Sn. Compared with SnO2 without Sb, the 1.06 μm laser reflection of ATO nano-particles doped with Sb is obviously lower. The reason is that by doping with Sb, the positive charge center caused by Sb5+ (0.60 nm) which replaced the position of Sn4+(0.69 nm) in SnO2 appears (the particles size decreases with the decrease of ionic radius), the excess electrons form the donor impurity level near the bottom of the conduction band, the carrier number greatly increases, and the plasmon effect generates, thus the near infrared (covering 1.06 μm laser) absorption increases greatly; with the increase of n(Sb)/n(Sn), the reflection follows the trend of decreasing first and then increasing. When the mole ratio of Sb to Sn is 2/10, the 1.06 μm laser reflection of ATO nano-particles decreases to a minimum. But when the content of Sb is too high, the acceptor level formed by Sb3+(0.76 nm) which replaced the position of Sn4+(0.69 nm) with the decrease of Sb5+ to Sb3+ ratio compensates part of electronics produced by Sb5+ (the particles size increases with the increase of ionic radius), then the effective carrier concentration decreases, thus the 1.06 μm laser reflection of ATO nano-particles is relatively high. With the increase of Sb content, the size of ATO nano-particles decreases first and then increases, the laser reflection changes with the size of ATO nano-particles, that is to say, the property is largely decided by the structure.
Table 1 Comparison of XRD data between standard SnO2 and ATO samples obtained with various mole ratios of Sb to Sn

Table 2 Lattice constant and cell volume along with different mole ratios of Sb to Sn

Fig. 5 Reflectivity curves of ATO powders obtained with various mole ratios of Sb to Sn
At the same time, the laser reflection values of ATO nano-particles with different Sb contents were tested by laser diode with the 1.06 μm emission wavelength. Table 3 displays the 1.06 μm laser reflection of ATO nano-particles with different Sb contents. The value of 1.06 μm laser reflection of ATO nano-particles tested by laser diode is similar to that of reflection tested by UV-visible spectroscopy, and the reflection law is accordant.
Table 3 1.06 μm laser reflectivity of ATO powders obtained with different mole ratios of Sb to Sn

4 Conclusions
1) The ATO nano-particles prepared by co-precipitation method have tetragonal rutile structure, with the spheroidal micro-morphology, and the particle size distribution range was several decade nanometers.
2) With the increase of Sb content, the lattice defect caused by Sb partial replacement of Sn hindered the growth rates of ATO grains. The grain size of ATO decreased, and the unit cell volume increased due to the lattice expansion.
3) Compared with the SnO2 particles doped without Sb, the 1.06 μm laser reflection of ATO nano-particles doped with Sb was obviously lower. Because of the influence of the changed structure, with the increase of Sb content, the reflection increased first, and then decreased.
4) When the the Sb content was 20%, 1.06 μm laser reflection of ATO nano-particles was below 0.02%, and the laser reflection performance achieved the best.
References
[1] WU Xiang-wei, CHEN Zhen-hua, HUANG Pei-yun. Influences of dehydrating process on properties of ATO nano-powders [J]. Transactions of Nonferrous Metals Society of China, 2004, 14(6): 1123-1128.
[2] ZHENG Min, WANG Bao. One-step synthesis of antimony-doped tin dioxide nanocrystallites and their property [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(2): 404-409.
[3] JIANG Ming-xi, YANG Tian-zu, GU Ying-ying, DU Zuo-juan, LIU Jian-ling. Preparation of antimony-doped nanoparticles by hydrothermal method [J]. Transactions of Nonferrous Metals Society of China, 2005, 15(3): 702-705.
[4] BENRABAH B, BOUAZA A, KADARI A, MAAREF A. Impedance studies of Sb doped SnO2 thin film prepared by sol gel process [J]. Superlattices and Microstructures, 2011, 50(6): 591-600.
[5] WANG Xin, HU Yuan, SONG Lei, XING Wei-yi, LU Hong-dian, LV Pin, JIE Gan-xin. Effect of antimony doped tin oxide on behaviors of waterborne polyurethane acrylate nanocomposite coatings [J]. Surface & Coatings Technology, 2010, 205(7): 1864-1869.
[6] SUN Dong-song, LIU Dong, XIA Hai-yun, WANG Bang-xin, ZHONG Zhi-qing, DONG Jing-jing, HU Huan-ling. Low tropospheric wind profile from a 1.06 μm doppler lidar [J]. Infrared and Laser Engineering, 2007, 36(1): 52-56.
[7] HUANG Xiao-gu, CHEN Jiao, ZHANG Jing, WANG Li-xi, ZHANG Qi-tu. A new microwave absorber based on antimony-doped tin oxide and ferrite composite with excellent electromagnetic match [J]. Journal of Alloys and Compounds, 2010, 506(1): 347-350.
[8] ZHU Bing-jie, ZHOU Feng, ZHANG Qing-hong, CHEN Li-yun, LI Yao-gang, WANG Hong-zhi. Solvothermal sysnthesis of Sb-doped SnO2 nanospheres and their properties [J]. Chinese Journal of Inorganic Chemistry, 2012, 27(2): 226-230. (in Chinese)
[9] SANTOS-PENA J, BROUSSE T. Antimony effecting on the electrochemical behavior of SnO2 thin film electrodes [J]. Power Sources, 2001, 4: 97-98.
[10] LIN Yang-yi, WU A T, KU C S, LEE H Y. Analysis of chlorine ions in antimony-doped tin oxide thin film using synchrotron grazing incidence X-ray diffraction [J]. Japanese Journal of Applied Physics, 51(10): 10NE28.
[11] LU H F, HONG R Y, WANG L S, XIE H D, ZHAO S Q. Preparation of ATO nanorods and electrical resistivity analysis [J]. Materials Letters, 2012, 68(1): 237-239.
[12] LEE S Y, PARK B O. Structural, electrical and optical characteristics of SnO2:Sb thin films by ultrasonic spray pyrolysis [J]. Thin Solid Films, 2006, 5(10): 154-158.
[13] YANG Bao-ping, ZHONG Xiao-hua, ZHANG Xiao-liang. Effects of Sb content on the structure and electric property of ATO nanoparticles [J]. Journal of Functional Materials, 2011, 42(11): 1993-1997.
[14] GONCHAR A G, RUD’ B M, BYKOV A I, SHELUD’KO V E, KREMENITSKYI V V. Effect of pressure–temperature treatment on the properties of antimony-doped tin dioxide [J]. Powder Metallurgy and Metal Ceramics, 2012, 51(3-4): 82-89.
[15] ZHONG Xiao-hua, YANG Bao-ping, ZHANG Xiao-liang, JIA Jun-hong, YI Ge-wen. Effect of calcining temperature and time on the characteristics of Sb-doped SnO2 nanoparticles synthesized by the sol–gel method [J]. Particuology, 2012, 10(3): 365-370.
Sb掺杂量对ATO纳米颗粒结构及激光吸收性能的影响
张 晶,王丽熙,梁敏鹏,张其土
南京工业大学 材料科学与工程学院,南京 210009
摘 要:以SnCl4·5H2O和SbCl3为原料,采用共沉淀法制备不同Sb掺杂量的氧化锡锑(ATO)纳米粉末。分别采用XRD、FESEM、紫外可见分光光度计和激光器对晶体结构、形貌、激光反射率进行表征,研究Sb掺杂量对ATO纳米颗粒的结构、晶粒尺寸和激光反射性能的影响。结果表明:共沉淀法制备的ATO纳米颗粒为四方相金红石结构,粒径大小约为几十纳米;随着Sb掺杂量的增加,ATO的晶粒尺寸减小,晶胞体积则逐渐增大;与未掺杂的SnO2粉末相比,Sb掺杂后的ATO粉末在1.06 μm激光波长处的反射率明显低于未掺杂的SnO2的反射率;随着Sb掺杂量的增加,反射率值呈先减小后增大的趋势,在Sb掺杂量为20 %时, ATO粉末在1.06 μm激光波长处的反射率低于0.02%,激光隐身性能最佳。
关键词:氧化锡锑(ATO);Sb掺杂量;共沉淀法;激光隐身
(Edited by Hua YANG)
Foundation item: Project (10KJB430008) supported by the Natural Science Foundation of Colleges in Jiangsu Province, China; Projects (2013(CXZZ13_0421), 2012(CXLX12_0425)) supported by Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), China; Research and Innovation Program for College Graduates of Jiangsu Province, China
Corresponding author: Qi-tu ZHANG; Tel: +86-25-83587246; E-mail: njzqt@126.com
DOI: 10.1016/S1003-6326(14)63038-7
Abstract: Antimony tin oxide (ATO) nano-particles doped with different Sb contents were prepared by co-precipitation method, using SnCl4·5H2O and SbCl3 as main raw materials. Microstructure, morphology and reflectivity curves were characterized by XRD, FESEM, UV-visible spectroscopy and laser, and the effects of Sb content on crystalline microstructure, crystal size and reflectivity curves of the ATO nano-particles were investigated systematically. The results show that the ATO nano-particles prepared by co-precipitation method have tetragonal rutile structure, with particle size distribution range of several decade nanometer. With the increase of Sb content, the grain size of ATO decreases, and the unit cell volume increases. Compared with the SnO2 particles without Sb, the 1.06 μm laser reflection of ATO nano-particles doped with Sb is obviously lower. With the increase of Sb content, the reflection increases first, then decreases; when the Sb content is 20 %, 1.06 μm laser reflection of ATO nano-particles is below 0.02%, and the laser reflection performance is the best.


