
Commercial AM60 alloy for semisolid processing: Alloy optimization and thermodynamic analysis
LI Yuan-dong(李元东)1, 2, D. APELIAN3, XING Bo(邢 博)1, MA Ying(马 颖)1, 2, HAO Yuan(郝 远)1
1. Key Laboratory of Gansu Advanced Nonferrous Metal Materials,
Lanzhou University of Technology, Lanzhou 730050, China;
2. Key Laboratory of Nonferrous Metal Alloys and Processing, Ministry of Education,
Lanzhou University of Technology, Lanzhou 730050, China;
3. Advanced Casting Research Center (ACRC), Metal Processing Institute (MPI), WPI,
Worcester, MA 01609, USA
Received 13 May 2010; accepted 25 June 2010
Abstract:
Semisolid processing is now a commercially successful manufacturing route to produce net-shape parts in automotive industry. The conspicuous results of alloy optimization with thermodynamic simulations for semisolid processing of commercial AM60 alloy were present. The results indicate that the available processing temperature range of AM60 alloy is 170 °C. The temperature sensitivity of solid fraction decreases with increasing solid fraction or with decreasing temperature above eutectic reaction temperature of AM60 alloy. When the solid fraction φs is 0.4, corresponding processing temperature is 603.8 °C and the sensitivity -dφs/dT is 0.0184. The effects of various alloying elements on the solidification behavior and SSM processability of AM60 alloy were calculated with Pandat software.
Key words:
AM60 alloy; semisolid processing; alloy optimization; thermodynamic analysis; solid fraction;
1 Introduction
With the development of research and practice of semisolid metal (SSM) processing, some semisolid processability criteria are proposed to predict or select, or even optimize the composition of an alloy for semisolid processing[1-3]. Recently, a new method based on thermodynamic simulation of phase diagram appears. The method proposed here provides a powerful tool for alloy selection and design[4]. And the simulation results also provide a useful guide for the experiments on semisolid processing.
In the past several years, some researchers[5] carried out some thermodynamic simulations on commercial aluminum alloys such as aluminum alloys 380, 319 and 206. Different alloying elements would have different influence on the solidification behavior, the temperature sensitivity of solid fraction, as well as the SSM temperature process window of these alloys. HAN and VISWANATHAN[6] investigated on tailoring the composition of A356/357 alloy to make it more suitable for SSM processing. Similarly, based on calculations of temperature sensitivity of the solid fraction, LIU and FAN[7-8] studied the suitability of commercial alloys with Al-Mg-Si, Mg-Al-Zn and Mg-Al-Mn systems for SSM processing, and they have identified that several alloys had great potential for thixo- and rheo-casting applications. Recently, a great deal of effort has been devoted to alloys optimization and design in semisolid processing[9-10].
In this work, the commercial AM60 alloy used for semisolid processing is optimized by means of thermodynamic simulations. This work is contributed to the optimal control of semisolid processing of magnesium alloy and favors the commercial production of semisolid magnesium components used in automobile industry.
2 Criteria of alloy selection for SSM processing
There was a fast progress in alloy selection, design, and optimization of SSM processing in the past few years. Meanwhile, the researchers generalized some criteria of alloy selection for SSM processing. The criteria of alloy selection are as follows:
1) Solidification temperature range (ΔT). It is usually defined as the temperature range between the solidus and the liquidus of an alloy. The solidification temperature range of pure metal and eutectic alloys is zero. Therefore, it is not suitable to select them for SSM processing. Whereas, alloys with too wide solidification temperature range are susceptible to hot tearing. So, it is suggested that the solidification range of an SSM alloy should be from 20 to 130 °C.
2) Temperature sensitivity of solid fraction (-dφs/dT). For a given alloy composition, the temperature sensitivity of solid fraction (φs) can be defined as the slope of solid fraction vs temperature curve and expressed as dφs/dT. Because dφs/dT is usually negative, so we can put a minus sign before it, i.e. -dφs/dT, to make it positive. This means that the temperature sensibility is the change of solid fraction per unit degree of temperature, so that it can result in the change of solid fraction. Therefore, for stability of φs that is required to be approximately constant in practice, a minimum -dφs/dT is favorable for industrial operations. LIU et al[7] proposed that the -dφs/dT should be less than 0.015 at the SSM processing temperature.
3) Temperature process window (ΔTTPW). It is also called operational temperature window. Because the temperature fluctuates during formation operation of specified alloys, a relatively large temperature window is expected. For a given alloy, the solid fraction φs is determined by processing temperature. In industrial practice, the ideal solid fraction φs should be from 0.3 to 0.5 for rheocasting, and from 0.5 to 0.7 for thixocasting/ thixoforging. Therefore, the temperature process window (ΔTTPW) is defined as the interval of temperature for solid fraction from 0.3 to 0.5 in the case of rheocasting, whereas, for thixocasting, it is defined as the interval of temperature when solid fraction is from 0.5 to 0.7.
Based on the above three criteria, therefore, we focus on the thermodynamic simulation for AM60 alloy in this work.
2.1 Solidification temperature range
Firstly, for the purpose of evaluation of semisolid processability of commercial AM60 alloy, its nominal composition is given in Table 1 on the basis of specification of ASTM, in which Al and Mn contents should take their middle values in the specified ranges. Therefore, all of the calculations are based on this composition to analyze the semisolid processability of AM60 alloy in use.
Table 1 Chemical composition of AM60 magnesium alloy (mass fraction, %)

From the result of calculations as shown in Fig.1, the solidification temperature range from liquidus to solidus is 231.81 °C (750.93-519.12 °C) for lever rule model and 346.64 °C (750.93-404.29 °C) for Scheil equation model. Moreover, the emergence of solid phase takes place at the same temperature of 750.93 °C. This emerging temperature of primary magnesium solid solution α(Mg) has only a slight difference between above-mentioned two models. It is 617.99 °C for lever rule and 617.97 °C for Scheil equation model. Along with the progression of solidification, this temperature difference of a subsequently emerging phase becomes more and more evident, such as the eutectic reaction plateau. The eutectic reaction plateau appears in Scheil equation model, but does not in lever rule model.
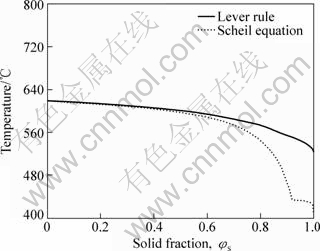
Fig.1 Comparison of temperature vs solid fraction curves obtained using lever rule and Scheil equation
Actually, the solidification temperature range does not govern the SSM processing in practice because the available solidification temperature range in SSM processing is smaller than the range from liquidus to solidus. Therefore, an available temperature range of solidification (ΔTATR) should be defined as the range of temperature from SSM processing to the end of eutectic solidification. The ΔTATR is an important parameter for SSM processing to determine the solidification behavior (also called secondary solidification behavior) such as secondary solidification phases, hot tearing, and pores. If the temperature of SSM processing is assumed as 603.83 °C (φs=0.4) and the eutectic reaction temperature as 433.37 °C for rehocasting, then the ΔTATR will be 170.46 °C by Scheil equation model.
2.2 Temperature sensitivity of solid fraction
As mentioned in the above discussion, temperature sensitivity of solid fraction is the most important parameter for well-controlled condition of temperature. An accurate temperature control, in fact, is very difficult during SSM processing. The smaller the -dφs/dT, the easier the control of processing temperature will be. Otherwise, a small temperature fluctuation can result in a large solid fraction variation. The variation of -dφs/dT with solid fraction and temperature are shown in Fig.2. As shown in Fig.2, the temperature sensitivity of solid fraction decreases with increasing fraction solid or with decreasing temperature when T>433.37 °C, the eutectic reaction temperature. When T=433.37 °C, a sharp increase in the sensitivity of solid fraction from 0.000 45 to 0.051 takes place, and then it decreases again with solid fraction till the end of solidification. Fortunately, the solid fraction of SSM processing is taken between 0.3 and 0.5 (for rehocasting) or between 0.5 and 0.7 (for thixocasting/thixoforging), so that the temperature of SSM processing, according to the solid fraction, is usually above the eutectic temperature, 433.37 °C. And as for rehocasting, we can choose 0.4 or so for solid fraction. Consequently, the processing temperature is 603.83 °C and -dφs/dT is 0.018 4.
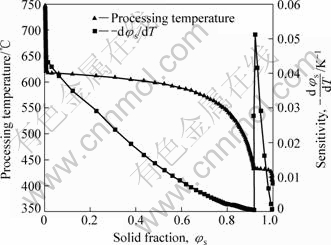
Fig.2 Relationship between solid fraction and sensitivity -dφs/dT, or processing temperature of AM60 alloy
2.3 Temperature process window (ΔTTPW)
Fig.3 shows the relationship between temperature process window and solid fraction of AM60 alloy to be used. Assume solid fraction φs to be 0.4 that is used in rehocasting, corresponding to processing temperature of 603.8 °C. Since φs for the available processing is from 0.3 to 0.5 in rehocasting, the processing temperature is given within the range of 597.4-608.6=11.2 °C.
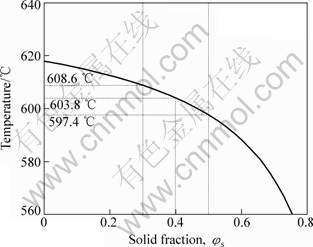
Fig.3 Relationship between temperature process window and solid fraction of AM60 alloy to be used
Fig.4 shows the relationship between the fraction solid and rehocasting temperature when the temperature process window is ±5 °C (10 °C) and ±10 °C(20 °C) for AM60 alloy. The variation of solid fraction vs rehocasting temperature for any temperature process window is ±5 °C or ±10 °C. Therefore, higher temperature is undesirable for AM60 alloy during semisolid processing.
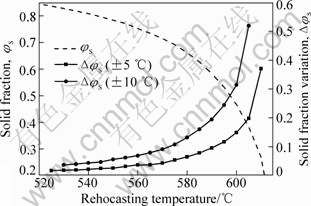
Fig.4 Relationship between fraction solid and rehocasting temperature when rehocasting temperature variation is ±5 °C and ±10 °C for AM60 alloy
3 Optimization of AM60 alloy for SSM processing
Pandat software package (version 7.0) was used for thermodynamic simulation of solidification process in non-equilibrium (Scheil equation) condition. In this work, we seek the optimal parameters in relation to AM60 alloy composition for semisolid processing.
Solid fraction is a critical parameter both for fundamental work and for control of the semisolid processing. For prediction of the relationship between solid fraction and temperature, the solidification characteristics are thus necessary for identification of alloy composition suitable for semisolid processing and analysis of the temperature sensitivity -dφs/dT of solid fraction to the temperature and temperature process window. It is necessary that the change of solid fraction does not too sharp with temperature during semisolid processing.
The composition of alloy is an original parameter of slurry properties. It is closely related with the solidification of alloy. Therefore, in order to understand the role of every alloying element and simplify the analysis, alloying elements other than the one under investigation are kept constant (as given in Table 1). Also, in order to increase the effect of each alloying element on the solidification behavior, the φs vs T curve, temperature sensitivity of the solid fraction, and content of alloying elements are to be varied within a range that extends to the limits specified by ASTM. Meanwhile, the alloying elements, Cu and Fe, are not analyzed in detail here because their contents are very low in AM60 alloy (Table 1), and actually they are taken as inclusions.
3.1 Effects of Al content
A series of curves of T-φs of AM60 alloy for different Al contents are predicted by using PANDAT/Scheil equation as shown in Fig.5, and some key parameters are obtained, as summarized in Fig.6.
Apparently, as the most important alloying element, Al has a significant influence on the solidification characteristics and consequently on the SSM processing ablility of the alloy.
From Fig.5 and Fig.6, we can see phenomena as follows. 1) The shape of the solid fraction vs temperature curve of AM60 alloy does not change with the variation of Al content. But it affects the start location of the binary eutectic point. With increasing Al content, the T-φs curve shifts toward the left, indicating that the amount of the binary eutectic reaction is increased. However, the temperature of the binary eutectic reaction does not change noticeably, and stands at about 433.37 °C. Moreover, the starting freezing temperature of the primary phase α-Mg is decreased with Al content increasing (Fig.5). As a result, the gradient of T-φs curve is increased, which implies that if a greater variation of temperature occurs, a small fluctuation will emerge subsequently. This is favorable for semisolid processing. 2) For the semisolid processing, the fraction solid is required within 0.3-0.5 (for rheocasting) or 0.5-0.7 (for thixocasting). In order to enhance process control, the Al content should be increased. Fig.6 indicates that the temperature of SSM processing is decreased with increasing Al content and fraction solid of the alloy. For example, when the solid fraction φs is constant as 0.4 (mostly for rheocasting) during semisolid processing, with the increase of Al content, the temperature can be decreased from 640.7 °C (1%Al) to 528 °C (15%Al), which is favorable for energy saving.
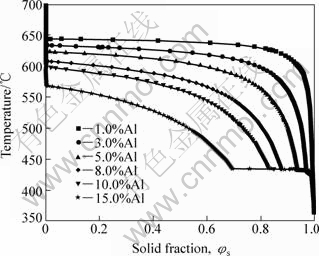
Fig.5 Effect of Al content on solid fraction vs temperature curves of AM60 alloy
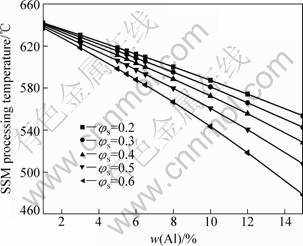
Fig.6 Effect of Al content on temperature for different solid fractions
The temperature sensitivity of solid fraction, -dφs/dT, depends on the alloy composition and the solid fraction. It is the slope of the solid fraction vs temperature curve. The process control requires a small value of -dφs/dT, while sophisticated heating system can control the temperature with a tolerance of ±0.5% of the processing temperature, and most industrial heating system gives an accuracy of about ±(1%-3%)[11]. Therefore, a minimum -dφs/dT is favorable in the solid fraction range of commercial operations. Fig.7 shows the temperature sensitivity as a function of solid fraction and Al content for AM60 alloy. With increasing the solid fraction in the range of semisolid processing (φs=0.3-0.7), the temperature sensitivity is reduced. It is more important that the temperature sensitivity is reduced with Al content at same solid fraction. That is to say, a greater Al content is common in SSM processing of AM60 alloy. But the increase of eutectic amount should be concerned.
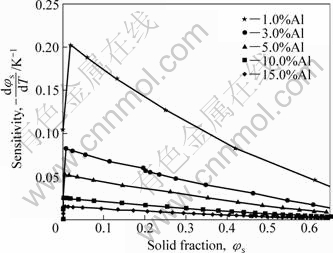
Fig.7 Temperature sensitivity as function of solid fraction and Al content for AM60 alloy
The variation of solid fraction is strongly affected by the semisolid processing temperature. In industrial practice, the variation of solid fraction is limited in a small range so that the SSM temperature process window is required. Therefore, for minimized variation of solid fraction, the SSM processing temperature can be controlled accurately.
Fig.8 shows that the temperature process window is calculated as a function of solid fraction and Al content for controlling target of solid fraction variation when it is limited in a range of ±0.05. For example, assuming φs=0.4 for SSM processing, the line, corresponding to φs=0.4, represents the temperature difference between φs=0.35 and φs=0.45. The temperature window ΔT increases with the Al content when φs is constant. The result suggests that it is beneficial to temperature process window by increasing the Al content when the φs is recommended.
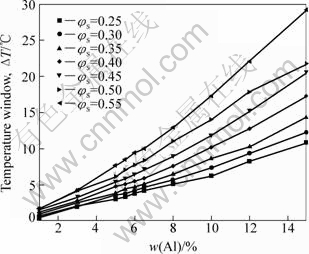
Fig.8 Temperature process window as function of Al content and solid fraction with maximum allowable solid fraction variation of ±0.05 for AM60 alloys
3.2 Effects of Mn content
For Mg-Al-Mn alloy when Mn content is less than 1% (mass fraction), the microstructure at ambient temperature usually consists of α(Mg)+β(Mg17Al12)+ MnAl. Besides, Mn is favorable which can reduce the disadvantage of Fe due to the lower corrosion resistance with Fe. Therefore, a lowest content of Mn is required for improving corrosion resistance of Mg-Al-Mn alloy[12]. So, the content of Mn is limited to 1%. However, the formation of Al-Mn compound will reduce the tendency of the formation of β-phase because Mn is added into binary Mg-Al alloys[13].
Fig.9 shows the solid fraction vs temperature curves for AM60 alloys with different Mn contents. These curves show a reversed order with respect to increasing Mn content to that with Al content in Fig.5 in which the T-φs curve shifts toward the left with increase of Al content. Fig.10 shows that the effect of Mn content on T-φs and temperature sensitivity of solid fraction -dφs/dT is slighter than that of Al content although the -dφs/dT increases with increasing Mn content. The variation of -dφs/dT is less than 0.01 when the Mn content increases from 0.1% to 4.0% (mass fraction). For a specified curve in Fig.10, such as the one with 0.4% Mn, the change of temperature from 595 °C to 605 °C results in a change of fraction solid φs from 0.539 to 0.392 (Δφs=0.147). According to above discussion, the lower Mn content is popular during SSM processing. Therefore, it is recommended that the lower limit of Mn content in the range specified by ASTM should be used for SSM processing.
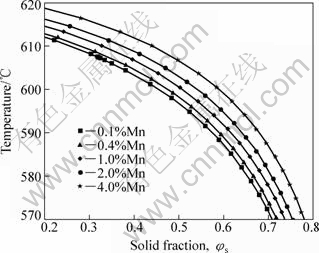
Fig.9 Solid fraction vs temperature curves for AM60 alloys with different Mn contents
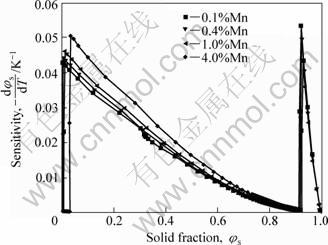
Fig.10 Effect of Mn content on temperature sensitivity of solid fraction
3.3 Effect of Zn content
Fig.11 gives the temperature vs solid fraction curves for AM60 alloy as a function of Zn content. It is shown that the temperature decreases for SSM processing with increasing the Zn content. And it is noticeable that the ternary eutectic emerges obviously when the Zn content is more than 1% (mass fraction). The ternary eutectic is a compound consisting of Mg, Al and Zn with low melting point. The compound with low melting point is often located at boundary of grains. As a result, mechanical properties of the material will decrease. PEKGULERYUZ and AVEDESIAN[14] reviewed the development of magnesium alloys, and explained the roles of Zn in magnesium alloy: on one hand, it plays a role of solid solution strengthening; on the other hand, the solubility of Al in Mg would be improved due to the Zn addition. But the hot tearing and brittleness follow when Zn content is beyond 1% (mass fraction).
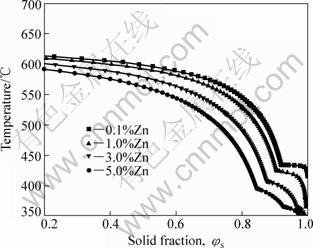
Fig.11 Temperature vs solid fraction curves for AM60 alloy as function of Zn content
Fig.12 shows the temperature sensitivity vs solid fraction curves as a function of Zn content. It is found that increasing Zn content decreases the temperature sensitivity in the solid fraction range typical for commercial SSM processing. Therefore, in order to get a relatively large SSM temperature process window, it is recommended to use the upper limit of Zn content specified by ASTM.
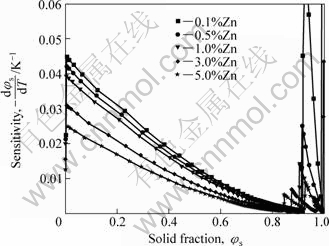
Fig.12 Temperature sensitivity vs solid fraction curves as function of Zn content
3.4 Effect of Si content
The Si content has a significant influence on the solidification of AM60 alloy, especially on the initial stage of solidification. The freezing temperature of Mg2Si increases significantly with increasing the Si content, and the order of phases solidifying in AM60 alloy is changed. Mg2Si is the fourth to be formed when Si content is less than 1% (mass fraction), while it is the second when Si content is 2%-3% (mass fraction), and it is the first when Si content is 5% (mass fraction). WANG et al[15] also found that the microstructure of AM60 cast alloy was effectively refined by adding small amount of Si. The grain size decreased from 180 μm to 80 μm with 1.8%Si addition, while the size increased with 2.5%Si addition[15]. So, Si is a dramatic element.
3.5 Phase transformation
The lever rule model is suitable only in ideal solidification of alloys. It is assumed that complete equilibrium is maintained between the solid and liquid phases. Extremely slow rate of cooling is required if this condition is to be approached. Hence, most alloys experience actually nonequilibrium solidification in practice. So, we have to use the Scheil equation model to analyze the solidification of AM60 alloy. Fig.13 shows the amount of each individual phase and temperature range over which it is formed. The main elements, such as Mg, Al, and Mn, are taken into account, whereas other elements are not thought since their contents are low. The primary solid phases are formed in solidification process, in which Al8Mn5, α-Mg(HCP), and Al11Mn4 are formed in order with decreasing the temperature. The eutectic reaction finishes to form the γ-Al12Mg17 in the end.
4 Conclusions
1) Commercial AM60 alloy has a wide solidification temperature range (346.64 °C) and an available temperature range if the temperature of SSM processing is assumed to be 603.83 °C (φs=0.4) and the eutectic reaction temperature of 433.37 °C for rehocasting, the ΔTATR=170.46 °C for Scheil equation model.
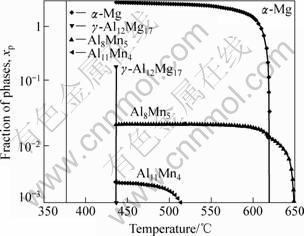
Fig.13 Calculated molar fractions of all solidified phases of AM60 alloy including main elements only
2) Primary formation of Al8Mn5, α-Mg(HCP), Al11Mn4, β2(BCC), Mg2Si and MgAlZn takes place during solidification process of AM60 alloy.
3) The sensitivity of solid fraction decreases with increasing solid fraction or increases with increasing processing temperature above the eutectic reaction temperature of AM60 alloy. When the solid fraction is around 0.4, the processing temperature is 603.83 °C, and -dφs/dT =0.0184.
4) Since the available processing φs is from 0.3 to 0.5 in rehocasting, the available range of processing temperature is 11.2 °C.
5) Al, Mn, Zn and Si have a significant influence on the SSM processability of AM60 alloy; therefore, it should be optimized for AM60 alloy to be used in SSM. Especially, Al affects the gradient of T-φs curve. So, it has the most significant effect on the processability of AM60 alloy. Based on the simulation results of AM60 alloy, an upper limit of Al content specified by ASTM is recommended.
Acknowledgements
One of the authors, LI Yuan-dong, would like to acknowledge financial support of China Scholarship Council (CSC) so that he has the opportunity to devote to the research at WPI.
References
[1] MACIEL C A, ATKINSON H V, KAPRANOS P, ARGENT B B. Thermodynamic predictions of wrought alloy compositions amenable to semi-solid processing [J]. Acta Materialia, 2003, 51: 2319-2330.
[2] POLA A, ROBERTI R, BERTOLI E, FURLONI D. Design and production of new aluminum thixotropic alloys for the manufacture of structural components by semisolid die casting [C]//KANG C G, KIM S K, LEE S Y. Proc of 9th Int Conf on Semi-solid Processing of Alloys and Composites. Busan, Korea, 2006: 58-63.
[3] WIESNER S, PAN Q Y, APELIAN D. Application of the continuous rheoconversion process (CRPTM) to low temperature HPDC—Part Ⅱ: Alloy development & validation [C]//KANG C G, KIM S K, LEE S Y. Proc of 9th Int Conf on Semi-solid Processing of Alloys and Composites. Busan, Korea, 2006: 64-67.
[4] DJURDJEVIC M B, SCHMID-FETZER R. Thermodynamic calculation as a tool for thixoforming alloy and process development [J]. Materials Science and Engineering A, 2006, 417(1/2): 24-33.
[5] FIGUEREDO A D. Science and technology of semi-solid metal processing [M]. Worcester: Worcester Polytechnic Institute, 2001: 410-415.
[6] HAN Q, VISWANATHAN S. The use of thermodynamic simulation for the selection of hypoeutectic aluminum-silicon alloys for semi-solid metal processing [J]. Materials Science and Engineering A, 2004, 364: 48-54.
[7] LIU Y Q, FAN Z, PATEL J. Thermodynamic approach to aluminium alloy design for semisolid metal processing [C]//TSUTSUI Y, KIUCHI M, ICHIKAMA K. Proceedings of the 7th S2P Advanced Semi-Solid Processing of Alloys and Composites. Tsukuba, Japan: National Institute of Advanced Industrial Science and Technology, 2002: 599-604.
[8] LIU Y Q, FAN Z. Magnesium alloy selections for semi-solid metal processing [C]//Proceedings of the 7th S2P Advanced Semi-Solid Processing of Alloys and Composites. Tsukuba, Japan: National Institute of Advanced Industrial Science and Technology, 2002: 587-592.
[9] PATEL J B, LIU Y Q, SHAO G, Fan Z. Rheo-processing of an alloy specifically designed for semi-solid metal processing based on the Al-Mg-Si system [J]. Materials Science and Engineering A, 2008,476(1/2): 341-349.
[10] LIU D, ATKINSON H V, JONES H. Thermodynamic prediction of thixoformability in alloys based on the Al-Si-Cu and Al-Si-Cu-Mg systems [J]. Acta Materialia, 2005, 53(14): 3807-3819.
[11] TZIMAS E, ZAVALIANGOS A. Materials selection for semisolid processing [J]. Materials and Manufacturing Processes, 1999, 14(2): 217-230.
[12] ZHANG Shi-chang, DUAN Han-qiao, CAI Qi-zhou, WEI Bo-kang, LIN Han-tong, CHEN Wei-chen. Effects of the main alloying elements on microstructure and properties of magnesium alloys [J]. Foundry, 2001, 50(6): 31-36. (in Chinese)
[13] LIU Y Q, DAS A, FAN Z. Thermodynamic predictions of Mg-Al-M(M Zn, Mn, Si) alloy compositions amenable to semisolid metal processing [J]. Materials Science and Technology, 2004, 20(1): 35-41.
[14] PEKGULERYUZ M O, AVEDESIAN M M. Magnesium alloying, some potentials for alloy development [J]. Journal of Japan Institute of Light Metals, 1992, 42(12): 679-686.
[15] WANG Li-guo, ZHANG Bao-feng, ZHU Shi-jie, ZHANG Mei, ZHANG Chun-xiang, GUAN Shao-kang. Effects of silicocalcium on microstructure and properties of Mg-6Al-0.5Mn alloy [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(3): 551-555.
Foundation item: Project(50964010) supported by the National Natural Science Foundation of China; Project(090WCGA894) supported by the International S&T Cooperation Program of Gansu Province, China
Corresponding author: LI Yuan-dong; Tel: +86-931-2976795; E-mail: liyd_lut@163.com
DOI: 10.1016/S1003-6326(09)60341-1


