文章编号:1004-0609(2008)S1-0070-04
亚熔盐法回收赤泥
钟 莉1, 2,张亦飞1
(1. 中国科学院 过程工程研究所,北京 100080;2. 中国科学院 研究生院,北京 100049)
摘 要:
采用亚熔盐法对赤泥中的Al2O3和Na2O的回收进行研究。实验第一步通过采用亚熔盐和添加适量石灰乳液回收赤泥中的Al2O3,使其中Al2O3的含量降至4.19%,Al2O3单程回收率达88%;实验第二步采用低浓度的NaOH溶液对赤泥进行深度脱钠,使得最终赤泥中Al2O3和Na2O的含量分别降到3.88%和1.39%,Al2O3/SiO2和Na2O/ SiO2相应降到0.17和0.06;经过回收Al2O3和Na2O后的终赤泥呈现出表面疏松多孔的颗粒状形貌。本研究在较低温的条件下综合回收赤泥中的Al2O3和Na2O。
关键词:
中图分类号:TQ 133.1 文献标识码:A
Recovery of Al2O3 and Na2O from red mud by sub-molten salt
ZHONG Li1, 2, ZHANG Yi-fei1
(1. Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100080, China;
2. Graduate University of Chinese Academy of Sciences, Beijing 100049, China)
Abstract: The recovery of Al2O3 and Na2O from red mud by sub-molten salt was investigated. In the first stage, via the application of a mixed system comprised of sub-molten salt and lime milk, the content of Al2O3 in the red mud was reduced to 4.19% and 88% of Al2O3 was recovered. In the second stage, the removal of Na2O was investigated by using NaOH solution with a low concentration. The results showed as follows: the content of Al2O3 in the final red mud with lacunaris configuration was reduced to 3.88%, the content of Na2O was reduced to 1.39%, Al2O3/SiO2 i.e. A/S, decreased to 0.17 and Na2O/SiO2 , i.e. N/S, decreased to 0.06. By the application of sub-molten salt, most Al2O3 and Na2O in red mud was recovered under low temperature.
Key words: red mud; sub-molten salt; alumina; sodium oxide
赤泥的回收与利用一直是氧化铝行业面临的世界性难题,目前全世界每年产生约5 000万t赤泥,我国的赤泥排放量每年大约也有400万t以上。如此大量的赤泥,国内外氧化铝厂大都将它们输送堆场、筑坝湿法堆存或干法堆存,长期占用大量土地,且含碱废液污染地表、地下水源,造成自然生态环境严重破坏,也制约着氧化铝生产的发展,赤泥综合回收与利用已成为发展氧化铝工业的重要课题。长期以来,国内外对赤泥的综合利用也进行了大量研究,包括赤泥中有用物质如钪[1]、铁的回收[2-4]、利用赤泥生产铸件[5]、建筑材料[6-7]及多种砖[8]、制成硅钙农用肥料以及吸附剂用于治理环境污染[9-16]等,但赤泥中有价紧缺资源氧化铝的回收一直是难点。目前,国内外已报道的有大规模工业应用价值的回收赤泥中氧化铝的工艺有水热法、氧化钙烧结法和高压水化学法,但水热法的溶出液中氧化铝的浓度很低,只能以水合铝酸钙的形式回收铝,难以与拜尔法主体溶出工序相配套,氧化钙烧结法(温度达1 000 ℃以上)氧化铝回收率高,但能耗太高而且渣量大,而占主导地位的高压水化学法氧化铝回收率高同时能回收一部分钠,但温度为260~280 ℃和压力达5.0 MPa以上,高温高压操作,导致生产能耗高,同时对设备要求相当高,操作难度大。本文采用亚熔盐法对赤泥的处理进行了研究,以克服上述工业化技术存在的高温高压操作导致设备投入高、工业操作难度大、能耗高等一系列问题,达到回收赤泥中的有价成分和消除赤泥带来的环境污染问题。
1 实验
实验的主要设备:机械搅拌不锈钢衬镍反应釜,容积0.5 L,d 80 mm,大连自动控制设备厂生产;CKW-Ⅲ温度控制仪,控制温度精度为±1 ℃,北京市朝阳自动化仪表厂生产。
实验原料为氧化铝生产过程产生的赤泥,其主要化学组成见表1。
表1 原料赤泥的主要化学成分
Table 1 Composition of red mud used in experiments
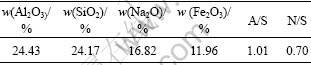
采用钠系亚熔盐法在低温低压条件下回收赤泥中的氧化铝后再回收氧化钠。
称取一定量的赤泥、亚熔盐、氧化钙和去离子水,混合均匀后转移至反应釜中,开通电源以及搅拌器冷却水,调节搅拌速度,设定控温程序后,将反应釜升温到设定温度反应一段时间,然后开通反应釜体冷却水结束反应,待温度降低至100 ℃后,开釜出料;料液趁热保温抽滤、液固分离,滤液即回收液取样后用酸溶液中和,再定容、稀释;滤渣即赤泥洗涤后于120 ℃左右烘干,然后熔融、稀释、定容。赤泥中氧化铝的回收率xA按下式计算:

回收液及赤泥中铝、硅、钠等含量采用ICP-OES (Optimal 5300DV, Perkin Elmer Instruments) 进行分析。固相分析采用扫描电镜和X射线衍所射仪 (X’Pert Pro MPD,Cu Kα 辐射,40 kV,30 mA)。
2 结果与讨论
实验研究了反应温度、亚熔盐的用量和CaO的添加量等对赤泥中氧化铝的综合回收效果的影响,结果如图1~3所示。
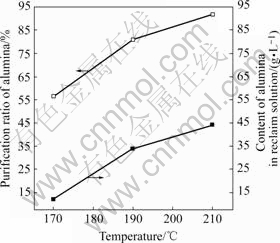
图1 反应温度对氧化铝回收效果的影响
Fig.1 Results of removal of alumina from red mud at different processing temperatures
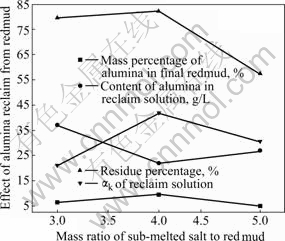
图2 亚熔盐用量对氧化铝回收效果的影响
Fig.2 Results of removal of alumina from red mud at different mass ratios of sub-melted salt to red mud
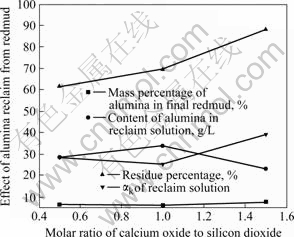
图3 CaO添加量对氧化铝回收效果的影响
Fig.3 Results of removal of alumina from red mud at different molar ratios of calcium oxide to silicon dioxide
2.1.1 反应温度对氧化铝综合回收效果的影响
图1表明,在实验范围170~210 ℃内,反应温度越高越有利于氧化铝的溶出回收,当反应温度为170 ℃时,氧化铝的回收效果很差,反应温度须选择在170 ℃以上,但同时要考虑温度越高能耗越大且反应介质对设备的腐蚀越严重等问题。
2.1.2 亚熔盐的用量(指亚熔盐与赤泥的质量比)对氧化铝综合回收效果的影响
实验用亚熔盐介质为质量分数为50%~70%的NaOH溶液。
图2显示,随着亚熔盐用量的不断增加,氧化铝的综合回收效果呈现先下降后上升的趋势,且亚熔盐与赤泥的质量比为5?1比3?1的效果好,但考虑到亚熔盐的用量越高,在溶出过程中循环的量就越大,循环效率就越低,希望溶出过程的亚熔盐的用量越低越好,因此宜选用亚熔盐的用量为3?1而不是5?1。
2.1.3 CaO的添加量(指CaO与SiO2的摩尔比)对氧化铝综合回收效果的影响
分析图3可知:随着CaO的添加量的升高,终泥中氧化铝含量变化不大,而渣率呈现不断上升趋势,回收液中氧化铝含量呈现先上升后下降趋势,回收液钠与铝摩尔比呈现先下降后上升趋势。综合考虑各指标,CaO与SiO2的摩尔比为1?1时Al2O3的综合回收效果较好。
通过以上正交实验及结果分析发现,实验范围内回收赤泥中Al2O3的最优条件为:反应温度为210 ℃,亚熔盐与赤泥的质量比为3?1,添加CaO与SiO2的摩尔比为1?1,反应时间4 h,搅拌转速700 r/min。在此条件下得到脱铝后的赤泥主要成分见表2;其中A/S比降至0.16,氧化铝的含量降至4.19%,单程回收率达88.05%。
表2 赤泥脱铝后的主要化学成分
Table 2 Composition of red mud after removal of alumina

对上述脱铝后的赤泥进行进一步脱钠。实验条件:温度为170 ℃,NaOH浓度为60 g/L,液固比为6,添加一定量的CaO,反应1~2 h。
得到脱铝脱钠后的赤泥主要化学组成、物相和型貌分析见表3、图4和图5。
表3 赤泥脱铝脱钠后的主要化学成分
Table 3 Composition of red mud after removal of alumina and sodium oxide

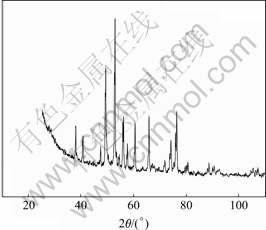
图4 赤泥脱铝脱钠后的XRD谱
Fig.4 XRD pattern of red mud after removal of alumina and sodium oxide
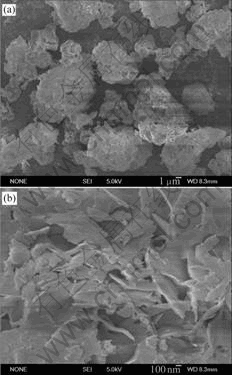
图5 赤泥脱铝脱钠后的SEM像
Fig.5 SEM images of red mud after removal of alumina and sodium oxide
从表3中看到:回收氧化铝和氧化钠后的终泥中Al2O3和Na2O的含量分别降到3.88%和1.39%,A/S和N/S比相应降到0.17和0.06。
由图4可知,终泥主要成分为Ca3Fe2(Si1.85Ti1.42O12)和Ca3(Fe0.87 Al0.13)2(SiO4)1.65(OH)5.4。
图5所示为最终所得赤泥在不同放大倍数下的SEM像。由图5可知,最终赤泥为表面多孔的薄片状叠加和堆积的非晶型颗粒状。多孔结构使得终赤泥具备制备成吸附剂、土壤改良剂的较大潜力,亟待进一步深入研究。
3 结论
1) 亚熔盐法回收赤泥中的氧化铝后,氧化铝含量由24.43%降至4.19%。
2) 先脱铝再脱钠后的终泥中Al2O3和Na2O的含量分别降到3.88%和1.39%,A/S从1.01降到0.17,N/S从0.70降至0.06。最终赤泥主要成分为钙铁镏石。
3) 通过亚熔盐法处理赤泥,达到了在较低温的条件下综合回收赤泥中的Al2O3和Na2O的目的。
REFERENCES
[1] MARIA T, HATZILE. Pilotplantinastigation of the leaching process for the recovery of scandia from red mud [J]. Industrial and Engineering Chemistry Research, 2002, 41(23): 5794- 5801.
[2] ANNA M, PAOLO P. New materials from industrial and mining wastes: Glass-ceramics and glass-and rock-wool fibre [J]. Int J Mineral Process, 1998(53): 121-134.
[3] MISHRA B, STDEY A. Recovery of value added products from red mud [J]. Minerals and Metallurgical Processing, 2002, 9(2): 87-89.
[4] JAMIESON E, JONES A, COOLING D, STOCKTON N. Magnetic separation of red sand to produce value [J]. Minerals Engineering,2006, 19(15): 1603-1605.
[5] SMITH N J, BUCHANAN V E, OLIVER G. The potential application of red mud in the production of castings [J]. Materials Science and Engineering A, 2006, 42(1/2): 250-253.
[6] VINCENZO M S, RENZO C. Bauxite ‘red mud’ in the ceramic industry (Part Ⅰ): Thermal behaviour [J]. Journal of the European Ceramic Society, 2000(20): 235-244.
[7] AMRITPHALE S S, ANSHUL A, CHANDRA N, RAMAKRISHNAN N. A novel process for making radiopaque materials using bauxite—Red mud [J]. Journal of the European Ceramic Society,2007, 27(4): 1945-1951.
[8] NEVIN Y, VAHDETTIN S. Utilization of bauxite waste in ceramie glazes [J]. Ceramics International, 2000(26): 485-493.
[9] WANG Xue-qian, NING Ping. The manufacture of H2S sorbent by using of the waste metallurgy [C]// International Conference of Clean Technologies for the Mining Industry. Santiage, Chile, 2000: 9-13.
[10] SONER A, SEMA A. Arsenic removal from aqueous solutions by adsorption on red mud [J]. Waste Management, 2000(20): 761-767.
[11] CENGELOGLU Y, TOR A, ERSOZ M, ARSLAN G. Removal of nitrate from aqueous solution by using red mud [J]. Separation and Purification Technology, 2006, 15(3): 374-378.
[12] TOR A, CENGELOGLU Y. Removal of congo red from aqueous solution by adsorption onto acid activated red mud [J]. Journal of Hazardous Materials,2006, 138(2): 409-415.
[13] GARAU G, CASTALDI P, SANTONA L, DEIANA P, MELIS P. Influence of red mud, zeolite and lime on heavy metal immobilization, culturable heterotrophic microbial populations and enzyme activities in a contaminated soil [J]. 2007, 142(1/2): 47-57.
[14] LIU Chang-jun, LI Yan-zhong, LUAN Zhao-kun, CHEN Zhao-yang, ZHANG Zhong-guo, JIA Zhi-ping. Adsorption removal of phosphate from aqueous solution by active red mud[J]. Journal of Environmental Sciences,2007, 19(10): 1166-1170.
[15] ZHU Chun-lei, LUAN Zhao-kun, WANG Yan-qiu and SHAN Xing-dong. Removal of cadmium from aqueous solutions by adsorption on granular red mud(GRM) [J]. Separation and Purification Technology,2007, 57(1): 161-169.
[16] YUNUS C, ALI T, GULSIN A. Removal of boron from aqueous solution by using neutralized red mud [J]. Journal of Hazardous Materials,2007, 142(1/2): 412-417.
基金项目: 国家重点基础研究发展计划资助项目(2007CB613501)
通讯作者:张亦飞,副研究员;电话:010-82610244;E-mail: yfzhang@home.ipe .ac.cn.
(编辑 陈爱华)


