DOI:10.19476/j.ysxb.1004.0609.2018.01.24
大相比鼓泡油膜萃取氰化废水中的低浓度金
刘 杰1,黄 焜1, 2,吴怀之1,刘会洲1, 2
(1. 中国科学院 青岛生物能源与过程研究所,中国科学院生物基材料重点实验室,青岛 266100;
2. 中国科学院 过程工程研究所,中国科学院绿色过程与工程重点实验室,北京 100190)
摘 要:
提出一种大相比鼓泡油膜萃取新方法,从氰化提金废水中萃取回收极低浓度金。考察季铵盐N263萃取体系大相比鼓泡油膜萃取过程的水相流量、油相流量、有机相负载量、有机相再生等因素对极低浓度金萃取回收的影响。结果表明:大相比鼓泡油膜萃取可高效、经济地回收氰化提金废水中的极低浓度金。萃余液中金浓度可降至0.01 mg/L以下,金萃取回收率达99%以上。低浓度金的萃取速率取决于水相流量、气泡表面包覆的有机萃取剂油膜层厚度、以及有机油膜层中的金属离子负载量等。
关键词:
文章编号:1004-0609(2018)-01-0199-06 中图分类号:TF831 文献标志码:A
黄金冶炼主要采用氰化浸出工艺,生产过程中往往会产生大量含金氰化废水,如氰化贫液、洗矿废水和尾矿浆等[1-3]。氰化物的强络合能力与剧毒性导致氰化提金废水成分复杂、处理难度大。现阶段为了满足环保要求,大部分黄金冶炼企业采用污水零排放工艺,产生的废水在冶炼厂内循环使用。但是,经多次循环后,铜、锌、铁等杂质离子浓度不断积累,尤其是SCN- 和Cu(CN)32-的积累会导致浸金液出现疲劳,浸金活性降低、氰化物消耗增大。因此,含氰废水有时不得不定期排放[4]。经检测,废水中含有浓度约为0.2~2.0 mg/L的极低浓度的金。此类废水如不加以处理而直接外排,不仅会对环境造成严重污染,还会造成黄金资源的严重浪费[5]。
目前,处理氰化提金废水的方法主要有氰化物破坏法和综合回收法。其中,综合回收法对于黄金企业降低成本、挖潜增效具有重要意义,主要包括活性炭吸附[6-8]、化学沉淀[9-10]、离子交换[11-13]等。化学沉淀法只是将污染物转移,极易造成二次污染,且试剂耗量大,无法经济回收极低浓度的金。吸附法和离子交换法效率低,饱和容量小,吸附速率慢,无法满足高处理量的需求。近年来,直接从氰化废液中选择性萃取金的研究已受到国内外越来越多的重视[14]。但是,传统的萃取技术在提取大体积废水中的极低浓度金氰络离子时,存在萃取剂分散损失严重、夹带量大、二次油污染、处理成本高等难题。如何经济、高效回收氰化废水中的极低浓度金已成为瓶颈难题[15]。
黄焜等[16-21]研发成功一种低浓度大相比鼓泡油膜萃取技术及配套装置。该技术利用有机萃取剂的表面活性,采用气泡分散极小体积的有机萃取剂,使极小体积的有机萃取剂分散包覆在气泡表面,形成一层萃取剂有机油膜。利用气泡表面的有机萃取剂油膜萃取富集极大体积水体中的极低浓度目标金属离子,萃取过程水油相比高达600以上,可实现极低浓度目标金属离子的高效萃取富集。该技术不同于传统的液膜萃取及离子气浮萃取,包覆在气泡表面的有机萃取剂油膜破坏容易,因引入气浮过程,萃余水相几乎无有机萃取剂夹带损失,不会产生二次油污染。采用该技术处理山东某黄金冶炼厂氰化提金废水的实验结果表明,该技术不仅可高效、经济回收大体积氰化废水中的极低浓度金氰络离子,富集比高,工艺成本低,而且可有效脱除氰化废水中的铜、锌、铁等杂质离子。萃取后排水可直接返回,在氰化厂内循环使用,无需排放,解决了氰化提金废水循环使用时,杂质离子积累的问题。本文作者以山东某黄金冶炼厂氰化提金废水为处理对象,采用低浓度大相比鼓泡油膜萃取新方法,详细考察了水相流量、油相流量、有机相负载量、有机相再生等因素对极低浓度金萃取回收的影响。
1 实验
1.1 试剂与材料
实验所用水相料液取自山东某黄金冶炼厂含金氰化废水,经测定水相初始pH为6~8,主要成份见表1。有机相为0.25mol/L的 N263(甲基三辛基氯化铵)煤油溶液。N263购自厦门先端科技有限公司;煤油(8008-20-6)购自中国石化总公司济南煤油厂;硫脲和盐酸购自国药集团化学试剂有限公司。
表1 废水中各离子成分的质量浓度
Table 1 Chemical analysis of ion concentrations in wastewater (mass concentration, mg/L)

1.2 实验设备
低浓度大相比鼓泡油膜萃取装置的结构原理示意图如图1所示。
水相料液自萃取装置的上端进水口注入,从下端出水口流出。从萃取装置的下端进气口泵入空气,然后从下端进油口泵入含有机萃取剂的油相。控制泵气流量和泵油流量,从萃取装置的下端喷口处喷出包覆一层有机萃取剂油膜的气泡(简称“油泡”)。控制泵油速率与泵气速率的相对比值,可控制油泡分散相表面包覆的有机萃取剂油膜层厚度。油泡分散相在萃取装置顶端的出油口聚并,负载有机相溢流出萃取装置。
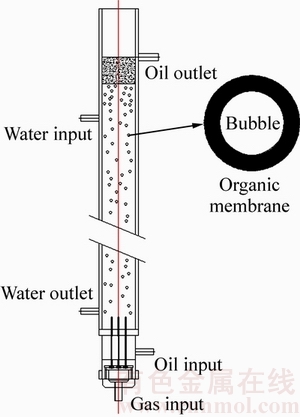
图1 低浓度大相比鼓泡油膜萃取装置
Fig. 1 Bubbling extraction column for gas-bubble supported organic liquid membrane extraction at large aqueous-to-oil phase ratios
1.3 实验方法
变动水相料液流量、油相流量、有机相负载量,并考察有机相反萃再生等条件对极低浓度金萃取回收的影响。
以萃取装置内开始产生稳定的油泡分散相时刻开始计时,分别收集从萃取装置顶端流出的有机相、以及从萃取装置底端流出的萃余水相,取样分析测定萃余水相中的金含量。每次取3个平行样,每个平行样10 mL。根据分析测定结果,计算金萃取回收率。
1.4 分析方法
萃余液中金离子的质量浓度采用美国安捷伦ICP-MS测定;pH值采用梅特勒台式酸度计S20测定。
2 结果与讨论
2.1 水相流量对萃取速率的影响
图2 给出了装置内水相流动和不流动两种状态下,萃余液中金残留浓度随时间的变化。
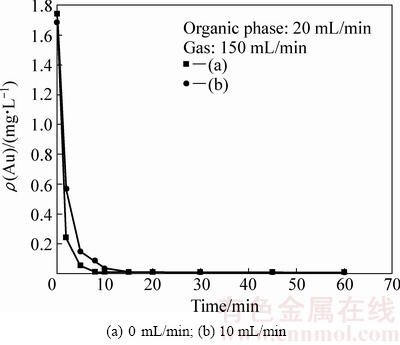
图2 水相流量对萃取速率的影响
Fig. 2 Effect of volume flowing rate of aqueous feed solutions on low-concentration gold extraction
由图2可知,水相不流动时,萃取8 min后,水相中的金氰络离子浓度即由初始的1.739 mg/L快速降至0.01 mg/L以下,金萃取回收率高于99%;当装置内水相流量为10 mL/min 时,萃取15min后才能达到平衡,水相中的金氰络离子浓度也可降至0.01 mg/L以下。显然,水相不流动时,萃余液中金氰络离子浓度下降速率较快。水相流动时,水相在装置内的停留时间缩短,油泡分散相与水相的相接触时间缩短。因此,达到同样萃取效果需要的时间也就越长。
此外,大相比鼓泡油膜萃取不同于传统的萃取方式。该方法利用有机萃取剂的表面活性,将极小体积的有机萃取剂分散包覆在微小气泡表面,在气泡表面铺展形成一层油膜薄层,由此可实现极小体积的有机萃取剂与水相接触界面积的最大化。萃取反应实际上是界面吸附的有机萃取剂分子与目标金属离子在界面发生相互作用,萃取速率取决于目标离子在油膜层界面的吸附行为。由于金氰络离子的离子半径大于铜氰络离子半径,其疏水性也大于铜氰络离子的。水相中的金氰络离子相比铜氰络离子,更容易在油膜薄层的相界面吸附,优先于铜氰络离子被萃取。因此,即使水相中的金氰络离子浓度极低,也能被高效萃取,并不受其他共存杂质离子的影响。
2.2 油相流量对萃取速率的影响
图3所示为当泵入萃取装置的水相流量不变、气相流量也不变的条件下,仅改变泵入油相流量,萃余液中金残留浓度随时间的变化。
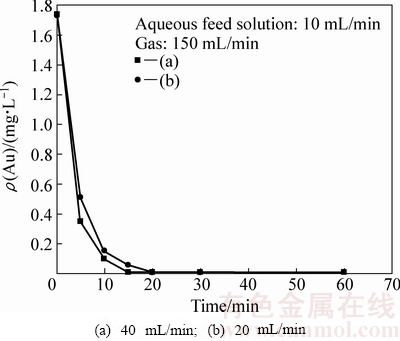
图3 油相流量对萃取速率的影响
Fig. 3 Effect of volume flowing rate of organic phase on low-concentration gold extraction
由图3可知,油相流量为20 mL/min的条件下,萃取20 min后,水相中金氰络离子的浓度即由初始的1.732 mg/L降至0.01 mg/L以下,金萃取回收率大于99%;但是,当油相流量提高到40 mL/min时,萃取仅15 min,水相中金氰络离子的浓度即快速降至0.01 mg/L以下。
实际上,当泵入萃取装置的水相流量不变、气相流量也不变时,在可形成稳定的油膜包覆气泡条件下,改变油相流量,将导致气泡表面包覆的油膜层厚度增加。油泡分散相上浮运动过程中,与连续逆流的水相剪切,油膜层越厚,越有利于油泡分散相表面的油膜层界面更新。因此,在可形成稳定的油膜包覆气泡条件下,油膜层越厚,萃取速率越快,萃余液中残留的金浓度下降越快。
2.3 有机相负载量对萃取速率的影响
采用N263进行大相比鼓泡油膜萃取时,除金氰络离子被萃取外,废水中的铜氰络离子等也会被萃取。图4给出了无负载的新鲜有机相与预先有一定负载的有机相连续泵入萃取装置时,萃余液中金残留浓度随时间的变化。
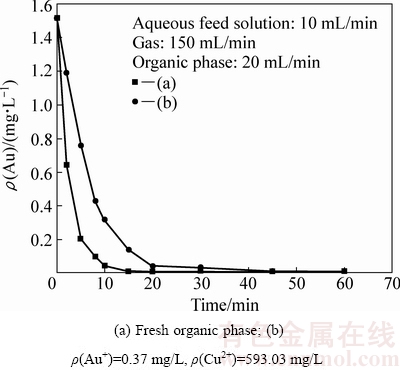
图4 有机相负载量对萃取速率的影响
Fig. 4 Effect of metal ion loading in organic phase on low-concentration gold extraction
由图4可知,当采用无负载的新鲜有机相萃取时,萃取15 min后,水相中的金氰络离子浓度即由初始的1.513 mg/L快速降至0.01 mg/L以下,金萃取回收率高于99%;但是,当采用预先有一定负载的有机相萃取同一种低浓度氰化提金废水时,萃取45 min后,水相中的残留金浓度才降至0.01 mg/L以下。显然,有负载的有机相对水相低浓度金的萃取速率明显低于无负载的新鲜有机相的。在鼓泡油膜萃取过程中,油泡分散相表面有机萃取剂油膜层萃取负载含量越大,将导致油膜层黏度增加,不利于油泡分散相表面的油膜层界面更新。因此,在可形成稳定的油膜包覆气泡条件下,有机相负载量越大,金萃取速率越慢。
2.4 有机相反萃再生循环使用对萃取效率的影响
用1.2 mol/L硫脲与1.5 mol/L盐酸的混合水溶液作反萃剂,在通空气的条件下,反萃负载了一定量金氰络离子和铜氰络离子的有机相,反萃时间0.5 h。结果表明,金的一次反萃率可达99%以上,铜的一次反萃率为94%。反萃后的有机相返回萃取使用。
图5对比了新鲜的未负载有机相、以及反萃再生后的有机相萃取氰化提金废水时,萃余液中金残留浓度随时间的变化。
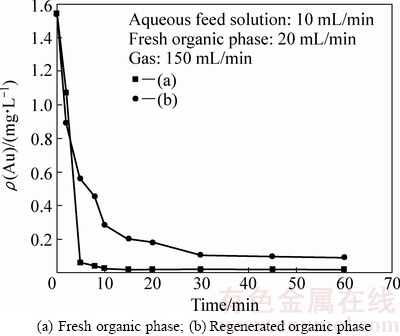
图5 有机相反萃再生对萃取效率的影响
Fig. 5 Effect of the regenerated organic phase for reusing on low-concentration gold extraction
由图5可知,反萃再生后的有机相萃取速率明显低于新鲜有机相。萃取45 min后,水相残留的金氰络离子浓度降至0.096 mg/L,且几乎不再变化,金萃取回收率为94%。使用经反萃再生后的有机相之所以萃取速率下降,可能系因有机相反萃未完全所致。与2.3节类似,油泡分散相表面的有机萃取剂油膜层有一定负载量时,金萃取速率下降。因此,反萃效率对于鼓泡油膜萃取效率影响较大。实际过程中,应尽量反萃取完全后,再重新返回使用。
实验也考察了将负载有机相经三级反萃后再返回使用的情况,证实了上述观点。萃取达平衡后,萃余液中金的残留浓度仍可降至0.01 mg/L以下。
目前,氰化厂的提金氰化废水一般要求返回使用,但废水中铜、锌、铁等杂质离子的积累将导致后续工艺效率降低,不得不定期排放。本研究表明,大相比鼓泡油膜萃取可高效回收氰化废水中的低浓度金氰络离子,并有效脱除氰化废水中的铜氰离子等杂质离子。萃取后的排水中的氰根离子浓度显著下降,可达标排放,也可在氰化厂内循环使用,无需排放,解决了氰化提金废水循环使用铜、锌、铁等杂质离子积累的问题。
3 结论
1) 大相比鼓泡油膜萃取技术可高效回收氰化提金废水中的极低浓度金氰络离子。即使废水中的金浓度<2 mg/L,且含有大量共存杂质离子,经大相比鼓泡油膜萃取后,萃余液金浓度仍可快速降至0.01mg/L以下,金萃取回收率可高达99%以上。
2) 鼓泡油膜萃取低浓度金的萃取速率受制于水相流量、在可形成稳定油膜包覆气泡条件下的油膜层厚度、以及有机相中金的负载量等因素。
3) 鼓泡油膜萃取得到的负载有机相反萃再生后,可反复循环使用。
REFERENCES
[1] BAS A D, KOC E, YAZICI Y E, DEVECI H. Treatment of copper-rich gold ore by cyanide leaching, ammonia pretreatment and ammoniacal cyanide leaching[J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 597-607.
[2] 周 军, 王丽君, 张 华. 含铁氰化提金废水综合回收研究[J]. 稀有金属, 2015, 39(10): 922-927.
ZHOU Jun, WANG Li-jun, ZHANG Hua. Recycling of cyanide leaching gold wastewater containing iron[J]. Chinese Journal of Rare metals, 2015, 39(10): 922-927.
[3] 高大明. 氰化物污染及其治理技术[J] .黄金, 1998, 19-25.
GAO Da-ming. Cyanide pollution and its control technology[J]. Gold, 1998, 19-25.
[4] 邢相栋, 兰新哲, 宋永辉, 张 静. 氰化法提金工艺中“三废处理技术”[J]. 黄金, 2008, 12(29): 55-60.
XING Xiang-dong, LAN Xin-zhe, SONG Yong-hui, ZHANG Jing. Disposal advances on “three wastes” treatment in the gold cyanidation extraction process[J]. Gold, 2008, 12(29): 55-60.
[5] 吴向阳, 于振福, 韩路波, 贾文涛. 活性炭吸附选矿废水中金等有价金属[J]. 黄金科学技术, 2013, 21(2): 51-54.
WU Xiang-yang, YU Zhen-fu, HAN Lu-bo, JIA Wen-tao. Recovery of valuable metals by active carbon adsorption from mineral processing wastewater[J]. Gold Science and Technology, 2013, 21(2): 51-54.
[6] ZHANG H, HUANG F, LIU D L, SHI P. Highly efficient removal of Cr(VI) from wastewater via adsorption with novel magnetic Fe3O4@C@ MgAl-layered double-hydroxide[J]. Chin Chem Lett, 2015, 26: 1137-1143.
[7] 伍喜庆, 黄志华. 改性活性炭吸附金的性能[J]. 中国有色金属学报, 2005, 15(1): 129-132.
WU Xi-qing, HUANG Zhi-hua. Adsorption of gold on modified activated carbon[J]. The Chinese Journal of Nonferrous Metals, 2005, 15(1): 129-132.
[8] KOSE T D, GHARDE A, MESHRAM N, GHARDE B, GHOLSE S. Application of mangifera indica seed shell for effective adsorption of Fe(II) and Mn(II) from aqueous solution[J]. Environmental Engineering and Management Journal, 2015, 14: 9-15.
[9] SCHWARTZ M O, PLOETHNER D. Removal of heavy metals from mine water by carbonate precipitation in the Grootfontein-Omatakocanal[J]. Environmental Geology, 2000, 39: 1117-1123.
[10] CHEN Q Y, LUO Z, HILLS C, XUE G, TYRER M. Precipitation of heavy metals from wastewater using simulated flue gas: Sequent additions of fly ash, lime and carbon dioxide[J]. Water Res, 2009, 43: 2605-2611.
[11] MOOSAVIRAD S M, SARIKHANI R, SHAHSAVANI E, MOHAMMADI S Z. Removal of some heavy metals from inorganic industrial wastewaters by ion exchange method[J]. Journal of Water Chemistry and Technology, 2015, 37: 191-198.
[12] 温俊杰, 张启修, 李 荐, 张贵清. 硅胶-聚合胺树脂从模拟低品位铜矿浸出液中富集纯化铜[J]. 中国有色金属学报, 2007, 17(1): 144-148.
WEN Jun-jie, ZHANG Qi-xiu, LI Jian, ZHANG Gui-qing. Enrichment and purification of copper from simulated leaching solution of low grade copper ores with silica gel-polyamine resin[J]. The Chinese Journal of Nonferrous Metals, 2007, 17(1): 144-148.
[13] LI J R, LI J R, YUAN B L, FU M L. Layered chalcogenide for Cu2+ removal by ion-exchange from wastewater[J]. J Mol Liq, 2014, 200: 205-302.
[14] 谢润芳, 杨项军, 韩云山, 李树华. 十二烷基二甲基苄基氯化铵从碱性氰化液中固相萃取金的研究[J]. 无机化学学报, 2014, 30(3): 487-491.
XIE Run-fang, YANG Xiang-jun, HAN Yun-shan, LI Shu-hua. Solid phase extraction gold from alkaline cyanide solution with benzyl dimethyl dodecyl ammonium chloride[J]. Chinese Journal of Inorganic Chemistry, 2014, 30(3): 487-491.
[15] 黄爱华. 提金含氰废水处理工艺研究现状及发展趋势分析[J]. 黄金科学技术, 2014, 22(2): 83-89.
HUANG Ai-hua. Analysis on the research status and development trend of treatment technology for gold smelting cyanide-containing wastewater[J]. Gold Science and Technology, 2014, 22(2): 83-89.
[16] 黄 焜, 刘会洲, 安振涛. 一种大相比液液两相连续萃取装置: 中国, 201110404565.2[P]. 2012-06-27.
HUANG Kun, LIU Hui-zhou, AN Zhen-tao. One kind of liquid-liquid two-phase continuous extraction equipment for operating at large aqueous-to-oil phase ratios: China, 201110404565.2[P]. 2012-06-27.
[17] 黄 焜, 肖传续, 刘会洲. 一种气泡表面有机液膜大相比萃取装置: 中国, 201410007908.5[P]. 2014-04-23.
HUANG Kun, XIAO Chuan-xu, LIU Hui-zhou. One kind of extraction equipment for operating at large aqueous-to-oil phase ratios based on organic liquid membrane covered bubbling extraction: China, 201410007908.5[P]. 2014-04-23.
[18] 黄 焜, 刘 杰, 吴怀之, 刘会洲. 一种气泡辅助的有机液膜发生器: 中国, 201410023670.5[P]. 2014-04-30.
HUANG Kun, LIU Jie, WU Hui-zhi, LIU Hui-zhou. One kind of organic liquid membrane generator assisted by gas bubbling: China, 201410023670.5[P]. 2014-04-30.
[19] 黄 焜, 肖传续, 郑 翰, 刘会洲. 一种水溶液中产生油膜包气泡分散相的装置及方法: 中国, 201410006753.3[P]. 2014-04-23.
HUANG Kun, XIAO Chuan-xu, ZHENG Han, LIU Hui-zhou. One kind of equipment and method for generating organic liquid membrane covered gas-in-oil bubbles: China, 201410006753.3[P]. 2014-04-23.
[20] HUANG K, LIU J, WU H Z, LIU H Z. A new bubbling extraction tower: toward liquid-liquid solvent extraction at large aqueous-to-oil phase ratios[J]. AIChE J, 2015, 61: 3889-3896.
[21] LIU J, HUANG K, WU H Z, LIU H Z. A feasible strategy for calculating the required mass transfer height of a new bubbling organic liquid membrane extraction tower directly based upon the experimental kinetic data[J]. Industrial & Engineering Chemistry Research, 2016, 55(16): 4426-4434.
Recovery of gold(I) with extremely low concentrations in cyanide wastewater by bubbling organic membrane extraction at large aqueous-to-oil phase ratios
LIU Jie1, HUANG Kun1, 2, WU Huai-zhi1, LIU Hui-zhou1, 2
(1. CAS Key Laboratory of Bio-based Materials, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao 266100, China;
2. CAS Key Laboratory of Green Process and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China;
Abstract: A new bubbling organic membrane extraction at large aqueous-to-oil phase ratios was suggested for recovery and enrichment of extremely low concentration gold(I) in cyanide wastewaters. The effect from the volume flowing rate of aqueous feed solution and organic phase, metal ion loading in the organic phase and the regenerated organic phase for reusing, on extraction of low concentration gold(I) during bubbling organic membrane extraction by quaternary ammonium salt N263, were investigated. Experimental results indicated that gold(I) cyanides with extremely low concentrations in wastewaters could be economically recovered and enriched efficiently by the suggested new method. Gold(I) concentration in the extraction raffinates could decrease to below 0.01mg/L, and the extraction recovery of gold(I) could reach above 99%. The extraction rate of gold(I) is subject to the volume flowing rate of aqueous feed solutions, the thickness of the organic liquid membrane layer on the surface of gas bubbles, and metal ion loading capacity in the organic membrane layer. The present work suggest a new approach for treatment of the wastewaters from gold cyanide processes.
Key words: solvent extraction; large aqueous-to-oil phase ratios; organic liquid membrane; cyanidation; low concentration; gold
Foundation item: Projects(51574213, 21606248) supported by the National Natural Science Foundation of China; Project(2013CB632602, 2012CBA01203) supported by the National Program on Key Basic Research Project, China
Received date: 2017-01-03; Accepted date: 2017-06-20
Corresponding author: HUANG Kun; Tel: +86-10-82544910; E-mail: khuang@ipe.ac.cn
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(51574213,21606248);国家重点基础研究发展计划资助项目(2013CB632602,2012CBA01203)
收稿日期:2017-01-03;修订日期:2017-06-20
通信作者:黄 焜,研究员,博士;电话:010-82544910;E-mail: khuang@ipe.ac.cn
摘 要:提出一种大相比鼓泡油膜萃取新方法,从氰化提金废水中萃取回收极低浓度金。考察季铵盐N263萃取体系大相比鼓泡油膜萃取过程的水相流量、油相流量、有机相负载量、有机相再生等因素对极低浓度金萃取回收的影响。结果表明:大相比鼓泡油膜萃取可高效、经济地回收氰化提金废水中的极低浓度金。萃余液中金浓度可降至0.01 mg/L以下,金萃取回收率达99%以上。低浓度金的萃取速率取决于水相流量、气泡表面包覆的有机萃取剂油膜层厚度、以及有机油膜层中的金属离子负载量等。
[2] 周 军, 王丽君, 张 华. 含铁氰化提金废水综合回收研究[J]. 稀有金属, 2015, 39(10): 922-927.
[3] 高大明. 氰化物污染及其治理技术[J] .黄金, 1998, 19-25.
GAO Da-ming. Cyanide pollution and its control technology[J]. Gold, 1998, 19-25.
[4] 邢相栋, 兰新哲, 宋永辉, 张 静. 氰化法提金工艺中“三废处理技术”[J]. 黄金, 2008, 12(29): 55-60.
[5] 吴向阳, 于振福, 韩路波, 贾文涛. 活性炭吸附选矿废水中金等有价金属[J]. 黄金科学技术, 2013, 21(2): 51-54.
[7] 伍喜庆, 黄志华. 改性活性炭吸附金的性能[J]. 中国有色金属学报, 2005, 15(1): 129-132.
[12] 温俊杰, 张启修, 李 荐, 张贵清. 硅胶-聚合胺树脂从模拟低品位铜矿浸出液中富集纯化铜[J]. 中国有色金属学报, 2007, 17(1): 144-148.
[14] 谢润芳, 杨项军, 韩云山, 李树华. 十二烷基二甲基苄基氯化铵从碱性氰化液中固相萃取金的研究[J]. 无机化学学报, 2014, 30(3): 487-491.
[15] 黄爱华. 提金含氰废水处理工艺研究现状及发展趋势分析[J]. 黄金科学技术, 2014, 22(2): 83-89.
[16] 黄 焜, 刘会洲, 安振涛. 一种大相比液液两相连续萃取装置: 中国, 201110404565.2[P]. 2012-06-27.
[17] 黄 焜, 肖传续, 刘会洲. 一种气泡表面有机液膜大相比萃取装置: 中国, 201410007908.5[P]. 2014-04-23.
[18] 黄 焜, 刘 杰, 吴怀之, 刘会洲. 一种气泡辅助的有机液膜发生器: 中国, 201410023670.5[P]. 2014-04-30.
[19] 黄 焜, 肖传续, 郑 翰, 刘会洲. 一种水溶液中产生油膜包气泡分散相的装置及方法: 中国, 201410006753.3[P]. 2014-04-23.


